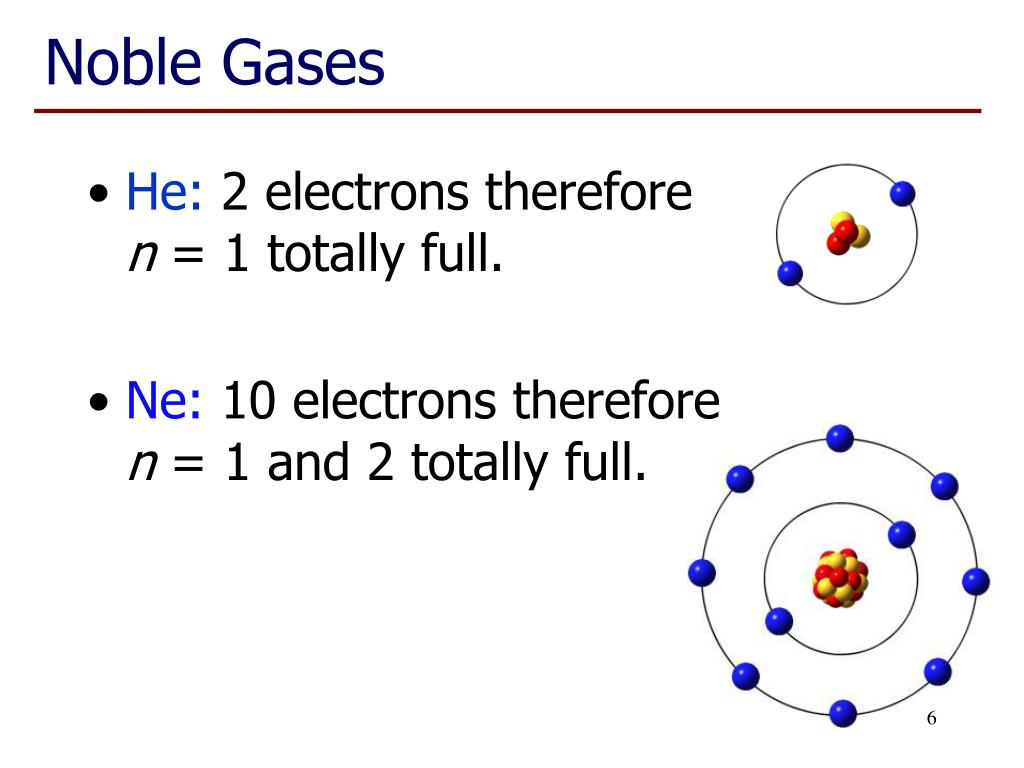
Do Noble Gases Form Ions
Do Noble Gases Form Ions - The elements are helium (he), neon (ne), argon (ar), krypton. Web noble gas, any of the seven chemical elements that make up group 18 (viiia) of the periodic table. Group 16 elements (two groups left) form 2− ions, and so on. Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble. Web under certain conditions, the noble gases can form diatomic gases, clathrates, fluorides, chlorides, metal complexes, and other compounds. Web noble gases (group 8): The noble gases are very unreactive. Part of chemistry (single science) atomic. Helium, neon, argon, kypton and xeon. Chemistry library > unit 7 lesson 4:
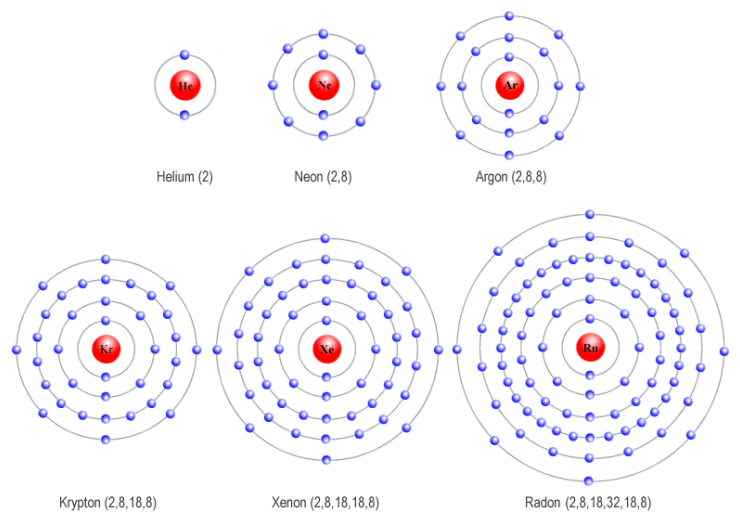
The noble gases of the periodic table do not have a charge because they are nonreactive. Web noble gases are odorless, colorless, nonflammable, and monotonic gases that have low chemical reactivity. Helium, neon, argon, kypton and xeon. The noble gases are very unreactive. 0 (uncharged) dianions, dications, and zwitterions. Web noble gases (group 8): Web the atoms of noble gases already have complete outer shells, so they have no tendency to lose, gain, or share electrons. Web what charge do noble gases take as ions? Web for example, group 17 elements (one group left of the noble gases) form 1− ions; This trend can be used as a guide in.
Web they are helium, neon, argon, krypton, xenon, and radon. Krypton and xeon form compounds only with. This trend can be used as a guide in. The noble gases are very unreactive. Web noble gases are odorless, colorless, nonflammable, and monotonic gases that have low chemical reactivity. Web for example, group 17 elements (one group left of the noble gases) form 1− ions; 0 (uncharged) dianions, dications, and zwitterions. Electron configurations shells, subshells, and orbitals introduction to electron configurations noble gas. There are special names for certain types of ions. Web here are five of the six noble gases:
Why do Noble Gases rarely form Bonds with other Atoms? MakeTheBrainHappy
Web noble gases are odorless, colorless, nonflammable, and monotonic gases that have low chemical reactivity. Web the atoms of noble gases already have complete outer shells, so they have no tendency to lose, gain, or share electrons. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed. The.
10 28 How Many Electrons Do Atoms Gain Lose
Web noble gases (group 8): Web here are five of the six noble gases: Web the ions have the electronic structure of a noble gas (group 0 element), with a full outer shell for elements in groups 6 and 7, the charge on the ion is equal to (8 minus group. Web noble gas, any of the seven chemical elements.
Trends on the Periodic Table Chemistry Is My Jam!
This trend can be used as a guide in. Group 16 elements (two groups left) form 2− ions, and so on. Web here are five of the six noble gases: A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed. The noble gases of the periodic table do.
Chemical Bonding
A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed. They're all colourless and transparent. Web they are helium, neon, argon, krypton, xenon, and radon. Web noble gases are odorless, colorless, nonflammable, and monotonic gases that have low chemical reactivity. For neutral atoms, the 3d orbitals are above.
What Are the Freezing, Melting, and Boiling Points of Solids, Liquids
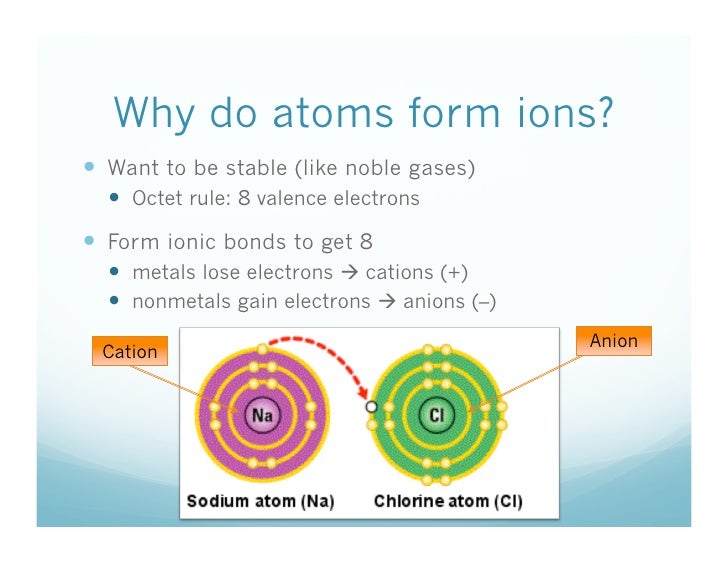
Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble. Web noble gases (group 8): Web gcse wjec group 0 and testing ions flame tests identify alkali metal ions in compounds. This trend can be used as a.
Ionic Compound Nomenclature Presentation Chemistry
For neutral atoms, the 3d orbitals are above the 4s orbitals in energy. Part of chemistry (single science) atomic. Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble. The elements are helium (he), neon (ne), argon (ar),.
Chemistry 9/15, 9/18 Noble Gas Configurations, The Atomic Museum
0 (uncharged) dianions, dications, and zwitterions. Chemistry library > unit 7 lesson 4: Web the atoms of noble gases already have complete outer shells, so they have no tendency to lose, gain, or share electrons. Part of chemistry (single science) atomic. Electron configurations shells, subshells, and orbitals introduction to electron configurations noble gas.
Ionic Compound Nomenclature Presentation Chemistry
The full valence electron shells of these atoms make. Web for example, group 17 elements (one group left of the noble gases) form 1− ions; Web noble gases are odorless, colorless, nonflammable, and monotonic gases that have low chemical reactivity. They're all colourless and transparent. Helium, neon, argon, kypton and xeon.
PPT Recap Atomic Structure PowerPoint Presentation, free download
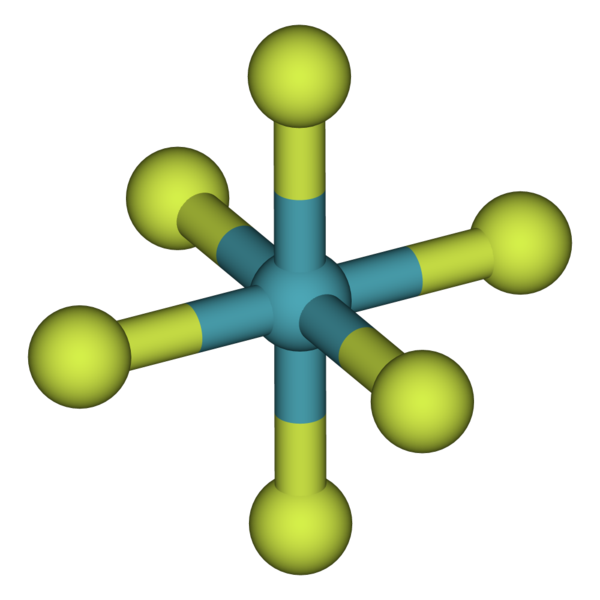
Electron configurations shells, subshells, and orbitals introduction to electron configurations noble gas. The full valence electron shells of these atoms make. This is why the noble gases are inert and do not take part. Web under certain conditions, the noble gases can form diatomic gases, clathrates, fluorides, chlorides, metal complexes, and other compounds. A noble gas configuration of an atom.
What Are Noble Gases? Definition and Properties
They're all colourless and transparent. Chemistry library > unit 7 lesson 4: Krypton and xeon form compounds only with. The full valence electron shells of these atoms make. Web for example, group 17 elements (one group left of the noble gases) form 1− ions;
Web Gcse Wjec Group 0 And Testing Ions Flame Tests Identify Alkali Metal Ions In Compounds.
Web noble gases (group 8): The full valence electron shells of these atoms make. Krypton and xeon form compounds only with. Electron configurations shells, subshells, and orbitals introduction to electron configurations noble gas.
Chemistry Library > Unit 7 Lesson 4:
A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed. Web here are five of the six noble gases: Web the ions have the electronic structure of a noble gas (group 0 element), with a full outer shell for elements in groups 6 and 7, the charge on the ion is equal to (8 minus group. Group 16 elements (two groups left) form 2− ions, and so on.
Web Ionic Bonding Is The Complete Transfer Of Valence Electron (S) Between Atoms And Is A Type Of Chemical Bond That Generates Two Oppositely Charged Ions.
Web noble gas, any of the seven chemical elements that make up group 18 (viiia) of the periodic table. The elements are helium (he), neon (ne), argon (ar), krypton. There are special names for certain types of ions. The noble gases of the periodic table do not have a charge because they are nonreactive.
They're All Colourless And Transparent.
0 (uncharged) dianions, dications, and zwitterions. Web what charge do noble gases take as ions? Web they are helium, neon, argon, krypton, xenon, and radon. Web for example, group 17 elements (one group left of the noble gases) form 1− ions;


.PNG)

.PNG)