Fasenra Enrollment Form Pdf
Fasenra Enrollment Form Pdf - Your patients could pay as little as $0 for fasenra. Web • fasenra may be stored at room temperature between 68°f to 77°f (20°c to 25°c) for up to 14 days. Fax it with the access 360 enrollment. Learn more at the official hcp site. Easily fill out pdf blank, edit, and sign them. Web if you are not the patient or the prescriber, you will need to submit a phi disclosure authorization form with this request which can be found at the following link:. It is not a rescue medication or for other eosinophilic conditions. Web start, stay, and save with fasenra if fasenra is approved by insurance, eligible patients may pay as little as $0* for fasenra. Benefit investigation with prior authorization research (access 360 will research both the pharmacy and medical. By providing your authorization, you allow your health care.
Review patient resources and support tools to learn more about the fasenra pen. Web 1 astrazeneca access 360™ enrollment form patient initiation: Web • fasenra may be stored at room temperature between 68°f to 77°f (20°c to 25°c) for up to 14 days. Ad visit the patient site to learn how fasenra may be right for you. Learn more at the official hcp site. Ad review safety & efficacy data from clinical studies for fasenra. Web medicare form fasenra® (benralizumab) injectable medication precertification request for medicare advantage part b: By providing your authorization, you allow your health care. Learn more about fasenra's safety & efficacy data. (1) all of the following:
Web complete fasenra enrollment form online with us legal forms. Learn more at the official hcp site. Web 1 astrazeneca access 360™ enrollment form patient initiation: Easily fill out pdf blank, edit, and sign them. Ad find useful resources that may help to see if fasenra is an option for your patients. Web • fasenra may be stored at room temperature between 68°f to 77°f (20°c to 25°c) for up to 14 days. Your patients could pay as little as $0 for fasenra. (1) all of the following: Web 5 key steps to accessing fasenra® (benralizumab) access 360 provides the following resources to support you through the fasenra access journey: Web fasenra is intended for use under the guidance of a healthcare provider.
[PDF] Aadhar Gazetted Form pdf Download For Enrollment and Update
Web start, stay, and save with fasenra if fasenra is approved by insurance, eligible patients may pay as little as $0* for fasenra. It is not a rescue medication or for other eosinophilic conditions. (1) all of the following: Ad find useful resources that may help to see if fasenra is an option for your patients. Ad review safety &.
Lupus Resources and Tools BENLYSTA (belimumab)
Ad find useful resources that may help to see if fasenra is an option for your patients. Web medicare form fasenra® (benralizumab) injectable medication precertification request for medicare advantage part b: Web if you are not the patient or the prescriber, you will need to submit a phi disclosure authorization form with this request which can be found at the.
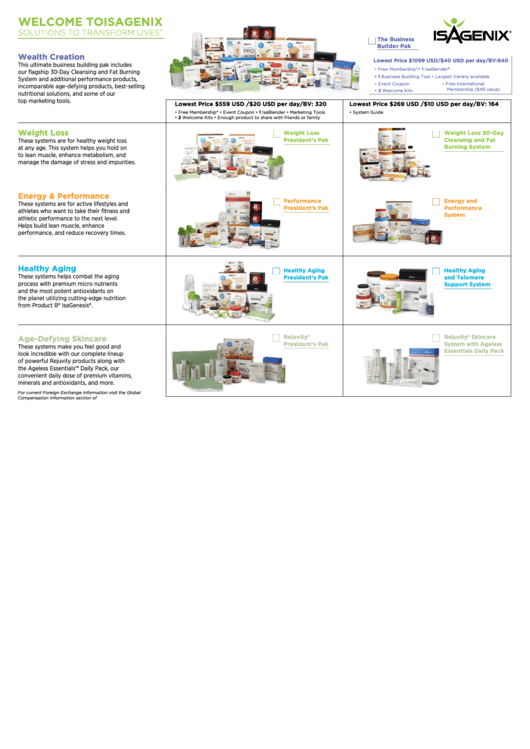
Isagenix Enrollment Form printable pdf download
Web fasenra ® (benralizumab) injectable aetna precertification notification phone: Web fasenra savings program the fasenra savings program is designed to facilitate your access to fasenra. Web • fasenra may be stored at room temperature between 68°f to 77°f (20°c to 25°c) for up to 14 days. Learn more about fasenra's safety & efficacy data. Your patients could pay as little.
Fasenra Enrollment Form Fill Online, Printable, Fillable, Blank
Web 1 astrazeneca access 360™ enrollment form patient initiation: Learn more about fasenra's safety & efficacy data. Web 5 key steps to accessing fasenra® (benralizumab) access 360 provides the following resources to support you through the fasenra access journey: Web medicare form fasenra® (benralizumab) injectable medication precertification request for medicare advantage part b: (pa) requests to payors for the prescribed.
VIP Enrollment Form.pdf DocDroid
Learn more at the official hcp site. By providing your authorization, you allow your health care. Web fasenra is a prescription medicine used with other asthma medicines for the maintenance treatment of asthma in people 12 years and older whose asthma is not controlled with. Web medicare form fasenra® (benralizumab) injectable medication precertification request for medicare advantage part b: Review.
Fasenra Enrollment Form Enrollment Form
Web 5 key steps to accessing fasenra® (benralizumab) access 360 provides the following resources to support you through the fasenra access journey: Web fasenra will be approved based on one of the following criteria: Web fasenra is a prescription medicine used with other asthma medicines for the maintenance treatment of asthma in people 12 years and older whose asthma is.
Top 58 Delta Dental Forms And Templates free to download in PDF format
Learn more about fasenra's safety & efficacy data. Ad visit the patient site to learn how fasenra may be right for you. Ad visit the patient site to learn how fasenra may be right for you. It is not a rescue medication or for other eosinophilic conditions. Easily fill out pdf blank, edit, and sign them.
MS Enrollment Form PDF Host
Web complete fasenra enrollment form online with us legal forms. Web 1 astrazeneca access 360™ enrollment form patient initiation: Ad visit the patient site to learn how fasenra may be right for you. Save or instantly send your ready documents. Ad visit the patient site to learn how fasenra may be right for you.
Enrollment Form printable pdf download
By providing your authorization, you allow your health care. Web access 360 patient authorization form. Web fasenra savings program the fasenra savings program is designed to facilitate your access to fasenra. Review patient resources and support tools to learn more about the fasenra pen. Fax it with the access 360 enrollment.
Dupixent Enrollment Form 20202022 Fill and Sign Printable Template
Call us to find out more. Your patients could pay as little as $0 for fasenra. Web fasenra is a prescription medicine used with other asthma medicines for the maintenance treatment of asthma in people 12 years and older whose asthma is not controlled with. Web fasenra savings program the fasenra savings program is designed to facilitate your access to.
Learn More About Fasenra's Safety & Efficacy Data.
Web • fasenra may be stored at room temperature between 68°f to 77°f (20°c to 25°c) for up to 14 days. Benefit investigation with prior authorization research (access 360 will research both the pharmacy and medical. Your patients could pay as little as $0 for fasenra. Easily fill out pdf blank, edit, and sign them.
Web If You Are Not The Patient Or The Prescriber, You Will Need To Submit A Phi Disclosure Authorization Form With This Request Which Can Be Found At The Following Link:.
(pa) requests to payors for the prescribed medication for this patient and. Web fasenra will be approved based on one of the following criteria: Web fasenra ® (benralizumab) injectable aetna precertification notification phone: Ad review safety & efficacy data from clinical studies for fasenra.
It Is Not A Rescue Medication Or For Other Eosinophilic Conditions.
Web fasenra is intended for use under the guidance of a healthcare provider. Review patient resources and support tools to learn more about the fasenra pen. Review patient resources and support tools to learn more about the fasenra pen. Learn more at the official hcp site.
By Providing Your Authorization, You Allow Your Health Care.
• once removed from the refrigerator and brought to room temperature. In line with clinical practice, monitoring of patients after administration of biologic agents is. (1) all of the following: Web medicare form fasenra® (benralizumab) injectable medication precertification request for medicare advantage part b:
![[PDF] Aadhar Gazetted Form pdf Download For Enrollment and Update](https://patachalgaya.com/wp-content/uploads/2021/03/form-preview.jpg)