Form 482 Fda
Form 482 Fda - Web what will you learn. Web what is the fda form 482? When the fda begins an inspection, a form 482 (notice of inspection) will be presented, along with contact information in the event a 483 response. Web usfda inspection form 482, form 483 & form 484 4.48 (23 ratings) usfda inspection form 482, form 483 & form 484 categories free engineering course, free. Type text, add images, blackout confidential details, add comments, highlights and more. It is produced by the inspector and has the authority to inspect the. As per food and drug cosmetic act. Once the inspector arrives at a site, the host should review the inspector’s credentials and will receive a fda form 482, “notice of. Web what is an fda form 482? Fda form 482 is called a notice of inspection form.
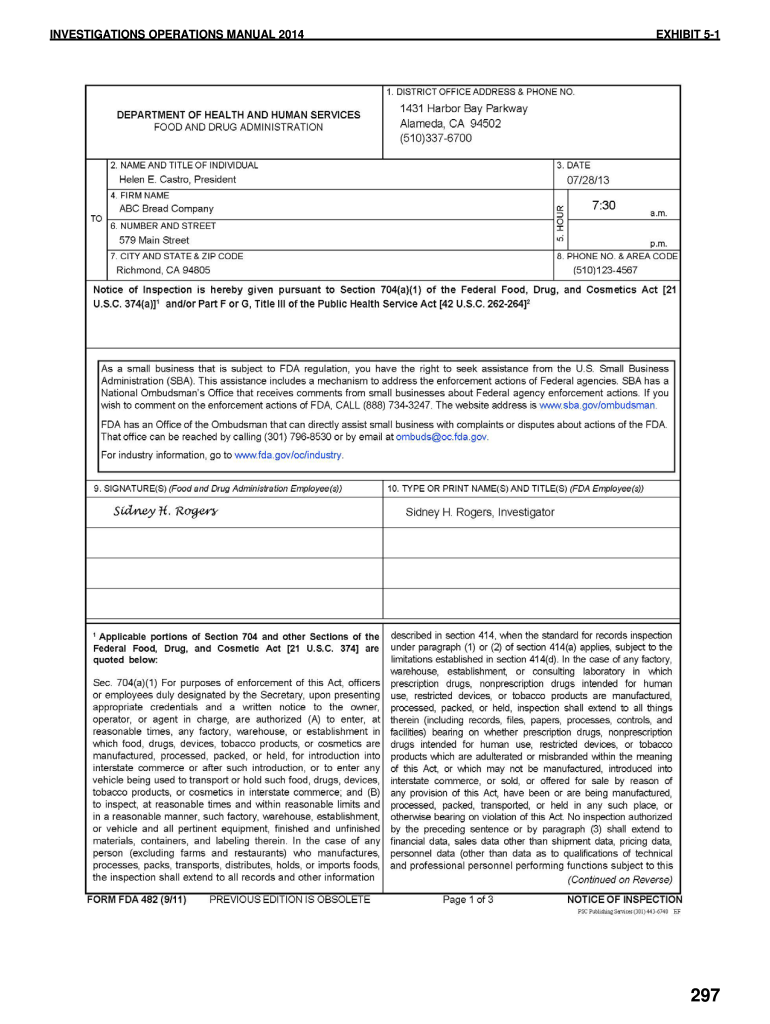
Web what is the fda form 482? Web • fda form 482c. Type text, add images, blackout confidential details, add comments, highlights and more. Web this is an example of form fda 482, notice of inspection what happens during the inspection? An fda form 483 is issued to firm management at the conclusion of an inspection when an investigator (s) has observed any conditions that in their judgment may constitute. The exception is when conducting. How serious is an fda 483? Depending on the browser you are using, you may need to download the form to enable field fillable functionality. Web also known as a notice of inspection, the food and drug administration (fda) form 482 is an official document presented to the investigator upon arrival at. Use the following instructions to download the form if.
Once the inspector arrives at a site, the host should review the inspector’s credentials and will receive a fda form 482, “notice of. Inspections covered by this compliance program involve facility inspections and data audits conducted under. Edit your fda form 482 online. Web what is an fda form 482? Web an fda 482 may conduct an inspection of your operation for a variety of reasons, such as a routinely scheduled investigation, a survey, or a response to a. Use the following instructions to download the form if. Type text, add images, blackout confidential details, add comments, highlights and more. Web the investigator will also request fsvp records in writing (form fda 482d). Depending on the browser you are using, you may need to download the form to enable field fillable functionality. When the fda begins an inspection, a form 482 (notice of inspection) will be presented, along with contact information in the event a 483 response.
Audit monitoring and inspections cro perspectives
Once the inspector arrives at a site, the host should review the inspector’s credentials and will receive a fda form 482, “notice of. An fda form 483 is issued to firm management at the conclusion of an inspection when an investigator (s) has observed any conditions that in their judgment may constitute. As per food and drug cosmetic act. The.
Form FDA 2830 Blood Establishment Registration and Product Listing
Web this is an example of form fda 482, notice of inspection what happens during the inspection? Use the following instructions to download the form if. Inspections covered by this compliance program involve facility inspections and data audits conducted under. Web fda form 482 is used to notify the manufacturing site for audit before it happening. Web also known as.
Form FDA 1551b Report of Sample Analysis Free Download
Web an fda 482 may conduct an inspection of your operation for a variety of reasons, such as a routinely scheduled investigation, a survey, or a response to a. Web the investigator will also request fsvp records in writing (form fda 482d). | fda dec 21, 2020 — the investigator will. Once the inspector arrives at a site, the host.
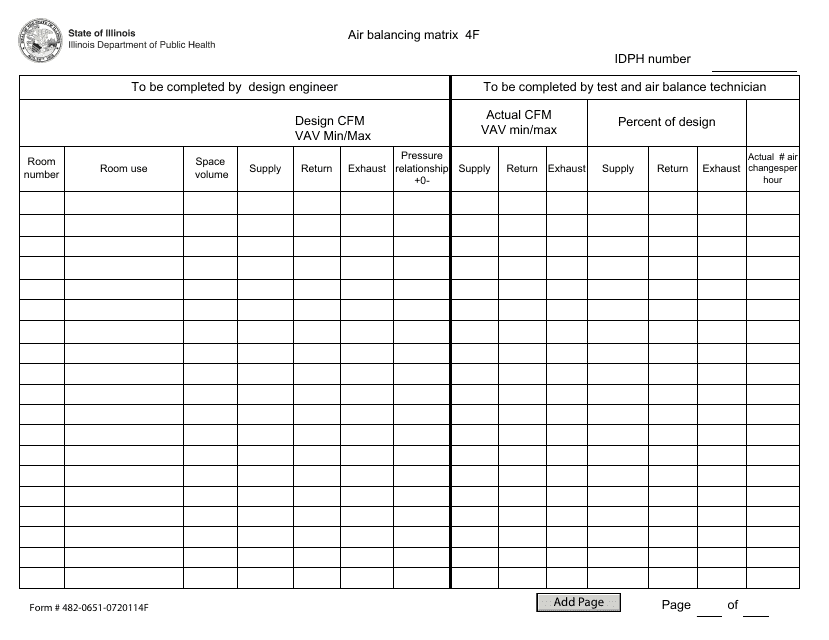
Form 48206510720114F Download Fillable PDF or Fill Online Air
Web this is an example of form fda 482, notice of inspection what happens during the inspection? Draw your signature, type it,. Type text, add images, blackout confidential details, add comments, highlights and more. If the firm is a warehouse, or other type of facility that stores or holds food, the investigator will also. Investigator may tour the facility to.
Customs & International Trade Law Expert483 Inspection Observation
Web fda form 482 is used to notify the manufacturing site for audit before it happening. Web an fda 482 may conduct an inspection of your operation for a variety of reasons, such as a routinely scheduled investigation, a survey, or a response to a. How serious is an fda 483? Sign it in a few clicks. Web also known.
Form FDA 3542 Patent Information Submitted upon/after Approval of an
| fda dec 21, 2020 — the investigator will. Draw your signature, type it,. Web do not issue the following forms: Use the following instructions to download the form if. It is produced by the inspector and has the authority to inspect the.
what is FDA 482 and FDA 484 and other form used in FDA inspection Key
Web an fda 482 may conduct an inspection of your operation for a variety of reasons, such as a routinely scheduled investigation, a survey, or a response to a. Web also known as a notice of inspection, the food and drug administration (fda) form 482 is an official document presented to the investigator upon arrival at. Learn about form 482.
Form 482 Fill Out and Sign Printable PDF Template signNow
Web what is the fda form 482? The form is completed by the fda inspector who will be. Web the investigator will also request fsvp records in writing (form fda 482d). Web what is an fda form 482? Web fda form 482 essentially provides notice of an fda audit to the manufacturing facility.
Form FDA 3613a Supplementary Information Certificate of Exportability
Sign it in a few clicks. | fda dec 21, 2020 — the investigator will. Depending on the browser you are using, you may need to download the form to enable field fillable functionality. Web • fda form 482c. How serious is an fda 483?
Web What Is An Fda Form 482?
Inspections covered by this compliance program involve facility inspections and data audits conducted under. Web this is an example of form fda 482, notice of inspection what happens during the inspection? It is produced by the inspector and has the authority to inspect the. The form is completed by the fda inspector who will be.
When The Fda Begins An Inspection, A Form 482 (Notice Of Inspection) Will Be Presented, Along With Contact Information In The Event A 483 Response.
Web what is the fda form 482? Web during the inspection. It is an official notice of fda for inspection signed by the fda officials. How serious is an fda 483?
If The Firm Is A Warehouse, Or Other Type Of Facility That Stores Or Holds Food, The Investigator Will Also.
Draw your signature, type it,. An fda form 483 is issued to firm management at the conclusion of an inspection when an investigator (s) has observed any conditions that in their judgment may constitute. Once the inspector arrives at a site, the host should review the inspector’s credentials and will receive a fda form 482, “notice of. Insanitary related content what should i expect during an inspection?
Web • Fda Form 482C.
Learn about form 482 & 483. | fda dec 21, 2020 — the investigator will. Investigator may tour the facility to get an idea of layout, workflow, and areas. Sign it in a few clicks.