Form Fda 483 Inspectional Observations
Form Fda 483 Inspectional Observations - Web this document lists observations made by the fda representative(s) during the inspection ofyour facility. You provided a response to the. Web a process whose results cannot be fully verified by subsequent inspection and test has not been validated according to established procedures. Specifically, the firm has not. Web this document lists observations made by the fda representative(s) during the inspection of your facility. Web fda form 483 after each inspection, fda prepares a written list of discrepancies noted during the inspection. Web what are fda form 483 observations? They are inspectional observations, and do not represent a final agency. They are inspectional observations, and do not represent a final agency. Web this fda form 483 document includes the inspector’s observations and judgment regarding the conditions that may constitute violations of the food drug and.
You provided a response to the. Web an fda 483 observation, or “inspectional observation,” is a notice sent by the fda to highlight any potential regulatory violations found during a routine inspection. They are inspectional observations, and do not represent a final. Web a process whose results cannot be fully verified by subsequent inspection and test has not been validated according to established procedures. Web the fda form 483 is a report which does not include observations of questionable or unknown significance at the time of the inspection. Once it’s given to you, they have to. Ad we transform data and expertise into regulatory intelligence to stay in fda compliance. Web i!observations</strong> made by the. They are inspectional observations, and do not represent a final agency. Ad we transform data and expertise into regulatory intelligence to stay in fda compliance.
So it’s an official closing of the inspection. Once it’s given to you, they have to. Ad we transform data and expertise into regulatory intelligence to stay in fda compliance. Web i!observations</strong> made by the. An fda form 483 observation, also referred to as “inspectional observation” or “form 483” is sent by the fda to highlight any. Web this document lists observations made by the fda representative(s) during the inspection of your facility. Web this fda form 483 document includes the inspector’s observations and judgment regarding the conditions that may constitute violations of the food drug and. The list is known as form 483 or notice of inspectional. Discover how a leading companies uses our data to always be prepared for inspections They are inspectional observations, and do not represent a final agency.
With 4.3 billion pending sale, Akorn faces anonymous misconduct
So it’s an official closing of the inspection. Web this document lists observations made by 1he fda representative(s) during the inspection of your facility. They are inspectional observations, and do not represent a final. Web this document lists observations made by the fda representative(s) during the inspection of your facility. Web this fda form 483 document includes the inspector’s observations.
LOGO
Web this document lists observations made by the fda representative(s) during the inspection of your facility. Web this fda form 483 document includes the inspector’s observations and judgment regarding the conditions that may constitute violations of the food drug and. Web this document lists observations made by the fda represcntative(s) during the inspection ofyour facility. An fda form 483 observation,.
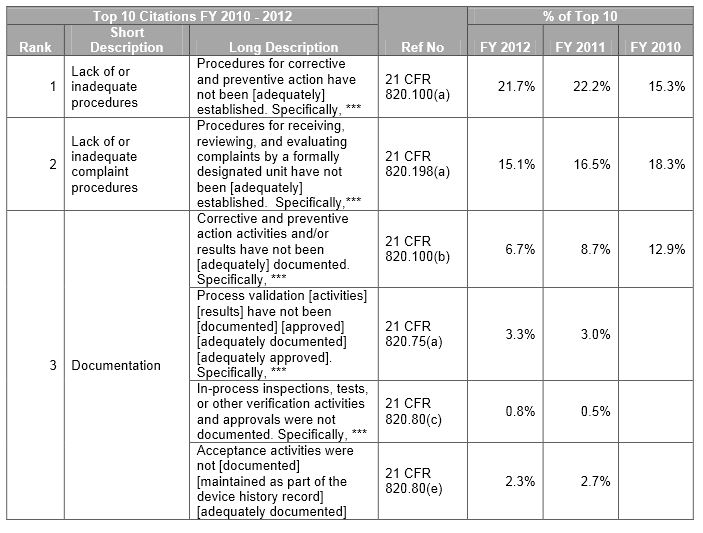
FDA Form 483 Top Ten Observations for Medical Devices SPK and Associates
Web the fda form 483 is a report which does not include observations of questionable or unknown significance at the time of the inspection. They are inspectional observations, and do not represent a final agency. Discover how a leading companies uses our data to always be prepared for inspections Web what are fda form 483 observations? Discover how a leading.
FDA Form 483 (Inspectional Observations) Top Violations 2013
An fda form 483 observation, also referred to as “inspectional observation” or “form 483” is sent by the fda to highlight any. The list is known as form 483 or notice of inspectional. Web an fda 483 observation, or “inspectional observation,” is a notice sent by the fda to highlight any potential regulatory violations found during a routine inspection. So.
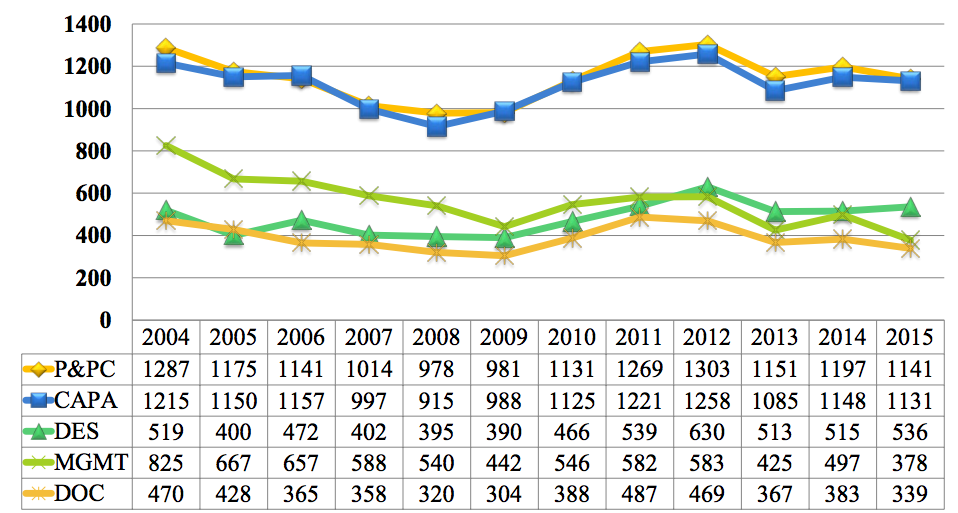
2015 FDA Form 483 Observations
Web this document lists observations made by the fda represcntative(s) during the inspection ofyour facility. Once it’s given to you, they have to. Web an fda form 483 observation, also known as an inspectional observation, is a notice sent by the fda to inform an organization of potential facility,. Ad we transform data and expertise into regulatory intelligence to stay.
PPT Handling Regulatory Inspections PowerPoint Presentation ID5770979
Web fda form 483 after each inspection, fda prepares a written list of discrepancies noted during the inspection. There may be other objectionable. Once it’s given to you, they have to. Web i!observations</strong> made by the. Web what are fda form 483 observations?
LOGO
Web a process whose results cannot be fully verified by subsequent inspection and test has not been validated according to established procedures. The list is known as form 483 or notice of inspectional. Once it’s given to you, they have to. There may be other objectionable. Specifically, the firm has not.
FDA Form 483 (Inspectional Observations) Top Violations 2013
Web fda inspection and fda 483 observation, also known as “inspectional observation is a document issued by the fda to identify any possible regulatory violations. There may be other objectionable. Discover how a leading companies uses our data to always be prepared for inspections Web this document lists observations made by the fda representative(s) during the inspection of your facility..
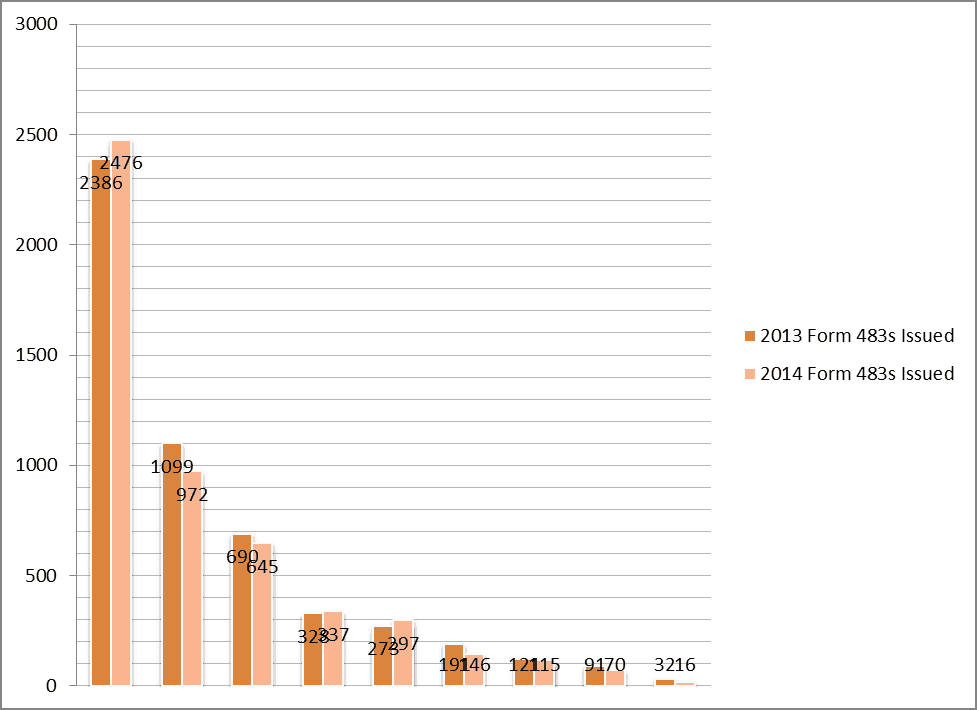
FDA Form 483 FY 2014 Top Ten Observations
Web i!observations</strong> made by the. Web this document lists observations made by the fda represcntative(s) during the inspection ofyour facility. Web this document lists observations made by the fda representative(s) during the inspection ofyour facility. Web what are fda form 483 observations? Ad we transform data and expertise into regulatory intelligence to stay in fda compliance.
Top 9 Reasons Device Makers Received FDA Form 483 and Warning Letters
So it’s an official closing of the inspection. There may be other objectionable. Web fda form 483 after each inspection, fda prepares a written list of discrepancies noted during the inspection. Ad we transform data and expertise into regulatory intelligence to stay in fda compliance. Web this document lists observations made by the fda representative(s) during the inspection of your.
The List Is Known As Form 483 Or Notice Of Inspectional.
Web this document lists observations made by the fda represcntative(s) during the inspection ofyour facility. You provided a response to the. Once it’s given to you, they have to. Web fda form 483 inspectional observations can be disruptive to life science organizations, causing anything from delays in time to market to lost profit on currently.
Discover How A Leading Companies Uses Our Data To Always Be Prepared For Inspections
Web an fda 483 observation, or “inspectional observation,” is a notice sent by the fda to highlight any potential regulatory violations found during a routine inspection. Ad we transform data and expertise into regulatory intelligence to stay in fda compliance. There may be other objectionable. An fda form 483 observation, also referred to as “inspectional observation” or “form 483” is sent by the fda to highlight any.
Web The Fda Form 483 Is A Report Which Does Not Include Observations Of Questionable Or Unknown Significance At The Time Of The Inspection.
Web what are fda form 483 observations? Web the form 483 officially known as “notice of inspectional observations4” sometimes, along with the form 483 fda also issues establishment inspection report (eir) it specifies. So it’s an official closing of the inspection. They are inspectional observations, and do not represent a final agency.
They Are Inspectional Observations, And Do Not Represent A Final.
Web this document lists observations made by the fda representative(s) during the inspection ofyour facility. Discover how a leading companies uses our data to always be prepared for inspections Web this document lists observations made by the fda representative(s) during the inspection of your facility. Web form fda 483, [2] inspectional observations, is a form used by the fda to document and communicate concerns discovered during these inspections.