How Many Covalent Bonds Can Hydrogen Form
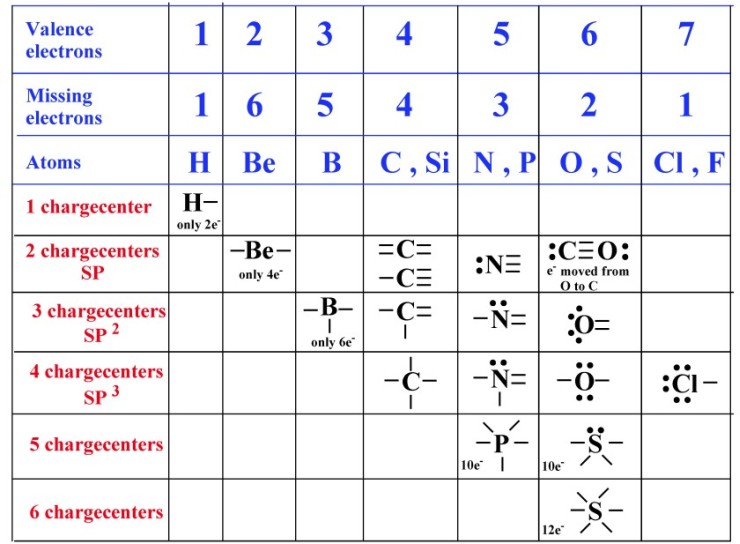
How Many Covalent Bonds Can Hydrogen Form - 1 shows the number of covalent. Hydrogen can form only single covalent bond. Web formation of covalent bonds. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. A hydrogen atom will typically form two covalent bonds because hydrogen has two electrons to. Web a covalent bond is a chemical bond that comes from the sharing of one or more electron pairs between two atoms. Web a hydrogen atom with one electron and a chlorine atom with 17 electrons after bonding, the chlorine atom is now in contact with eight electrons in its outer shell, so it is stable. How many covalent bonds do carbon nitrogen. Web a hydrogen atom will typically form two covalent bonds because hydrogen has two electrons to share in a bond. Oxygen can form double covalent bond.
For example, the hydrogen molecule, h 2, contains a covalent bond. Web a covalent bond is formed between two atoms by sharing electrons. Oxygen can form double covalent bond. Web a hydrogen atom with one electron and a chlorine atom with 17 electrons after bonding, the chlorine atom is now in contact with eight electrons in its outer shell, so it is stable. How many covalent bonds can hydrogen form? Web 02/06/2017 chemistry high school answered a hydrogen atom has one electron. Web formation of covalent bonds. Two with the hydrogen atoms and two with the with. 1 shows the number of covalent. Web how many covalent bonds can hydrogen form?
Web how many covalent bonds will a hydrogen atom normally make? Hydrogen can form only single covalent bond. Web how many covalent bonds can hydrogen form? Web formation of covalent bonds. Web hydrogen can form one covalent bond to have two valence electrons. How many covalent bonds do carbon nitrogen. This is the reason why h is always a terminal atom and never a central atom. Oxygen can form double covalent bond. A proposed explanation for a set of observations. Hydrogen and helium are notable exceptions to the general rule that atoms share an appropriate.
How many covalent bonds can hydrogen, oxygen, nitrogen and carbon form
A proposed explanation for a set of observations. Hydrogen is an example of an extremely simple. Terms in this set (28) one covalent bond a hydrogen atom has one electron. Web how many covalent bonds can hydrogen form? Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent.
Covalent Bonding (Biology) — Definition & Role Expii
Web hydrogen only needs to form one bond. A solution with a ph of 7 is. Hydrogen can form only single covalent bond. Covalent bonds can occur with most elements on the. For example, the hydrogen molecule, h 2, contains a covalent bond.
[Best Answer] How many covalent bonds does carbon form if each of its
Web a hydrogen atom with one electron and a chlorine atom with 17 electrons after bonding, the chlorine atom is now in contact with eight electrons in its outer shell, so it is stable. 1 shows the number of covalent. Both covalent and hydrogen bonds are forms of intermolecular forces. Oxygen can form double covalent bond. A solution with a.
Is SiO2 Ionic or Covalent? Techiescientist
In the formation of a covalent hydrogen molecule, therefore, each hydrogen atom forms a single bond,. Web hydrogen only needs to form one bond. Web how many covalent bonds can hydrogen form? Web a covalent bond is formed between two atoms by sharing electrons. Oxygen can form double covalent bond.
2.2 Chemical Bonds Anatomy & Physiology
Web a hydrogen atom will typically form two covalent bonds because hydrogen has two electrons to share in a bond. Web formation of covalent bonds. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: The number of bonds an element forms in a covalent compound is determined by the number of electrons it..
how many bonds does sulfur form
It all depends on their covalency. Terms in this set (28) one covalent bond a hydrogen atom has one electron. The number of bonds an element forms in a covalent compound is determined by the number of electrons it. Web how many covalent bonds can hydrogen form? Two with the hydrogen atoms and two with the with.
Bonds That Hold Water Molecules Together / Intermolecular Forces
Hydrogen can form only single covalent bond. Web a covalent bond is a chemical bond that comes from the sharing of one or more electron pairs between two atoms. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons;.
Covalent Bonds Biology for NonMajors I
Hydrogen is an example of an extremely simple. Oxygen can form double covalent bond. A hydrogen atom will typically form two covalent bonds because hydrogen has two electrons to. Terms in this set (28) one covalent bond a hydrogen atom has one electron. Such a bond is weaker than an ionic bond or.
HONC 1234 ChemSimplified
How many covalent bonds do carbon nitrogen. Web 02/06/2017 chemistry high school answered a hydrogen atom has one electron. Hydrogen is shown in fig 2.28 with one electron. Web a hydrogen atom will typically form two covalent bonds because hydrogen has two electrons to share in a bond. It all depends on their covalency.
Ch4 Polar Or Nonpolar Covalent Bond A CH4 B H2O C CF4 D CH3F Non
How many covalent bonds can hydrogen form? This is the reason why h is always a terminal atom and never a central atom. Nitrogen can form triple covalent bond. Two with the hydrogen atoms and two with the with. Both covalent and hydrogen bonds are forms of intermolecular forces.
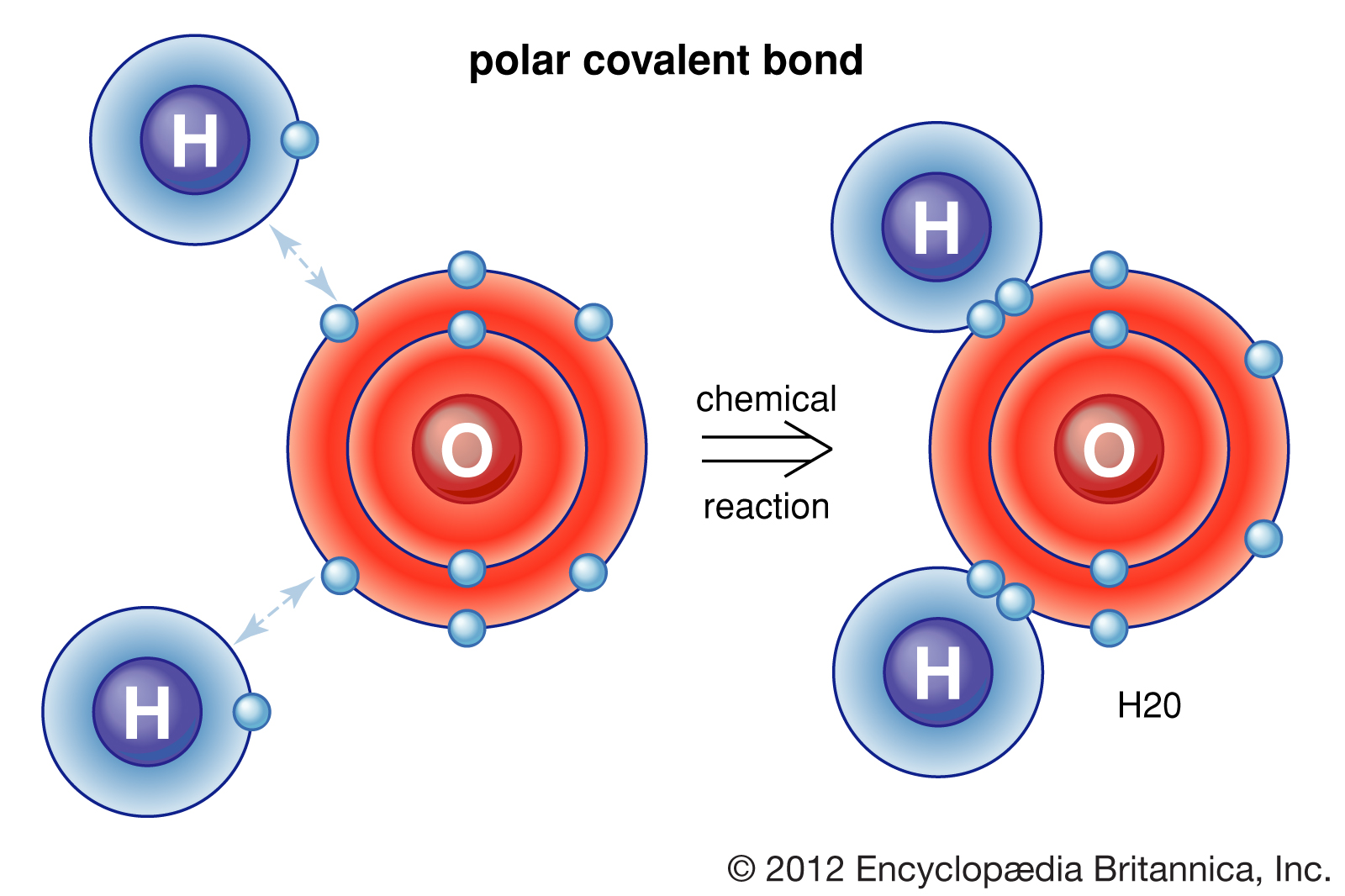
Web A Water Molecule Consists Of Two Hydrogen Atoms Bonded To An Oxygen Atom, And Its Overall Structure Is Bent.
Web a covalent bond is formed between two atoms by sharing electrons. Covalent bonds can occur with most elements on the. Two with the hydrogen atoms and two with the with. Web how many covalent bonds can hydrogen form?
Web A Hydrogen Atom With One Electron And A Chlorine Atom With 17 Electrons After Bonding, The Chlorine Atom Is Now In Contact With Eight Electrons In Its Outer Shell, So It Is Stable.
Web how many covalent bonds will a hydrogen atom normally make? The number of bonds an element forms in a covalent compound is determined by the number of electrons it. A hydrogen atom will typically form two covalent bonds because hydrogen has two electrons to. Hydrogen is shown in fig 2.28 with one electron.
Web A Covalent Bond Is A Chemical Bond That Comes From The Sharing Of One Or More Electron Pairs Between Two Atoms.
1 shows the number of covalent. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Nitrogen can form triple covalent bond. Hydrogen and helium are notable exceptions to the general rule that atoms share an appropriate.
Web 1 / 28 Flashcards Learn Test Match Created By Summer_32 Project For Last Week Of Quarter 2.
Web hydrogen only needs to form one bond. In the formation of a covalent hydrogen molecule, therefore, each hydrogen atom forms a single bond,. Web a hydrogen atom will typically form two covalent bonds because hydrogen has two electrons to share in a bond. It all depends on their covalency.

![[Best Answer] How many covalent bonds does carbon form if each of its](https://us-static.z-dn.net/files/df7/e45f4693a936673d74acd687e2bb88cf.png)