Nitrogen Gas And Hydrogen Gas React To Form Ammonia
Nitrogen Gas And Hydrogen Gas React To Form Ammonia - Web nitrogen gas (n₂) reacts with hydrogen gas (h₂) to form ammonia (nh₃). Asked • 03/19/18 in the reaction of nitrogen gas with hydrogen gas to form ammonia gas, how many grams of hydrogen are needed to produce 1.80. Web counting atoms in chemical equations nitrogen gas reacts with hydrogen to produce ammonia. What volume of ammonia would be produced by this reaction if 8.8 m² of nitrogen were consumed? Web chemistry chemistry questions and answers 161 14. Web this reaction is the synthesis of ammonia using nitrogen and hydrogen gas. Use the chemical equation to complete the statements. At 200°c in a closed container, 1.00 atm of nitrogen gas is mixed with 2.00 atm of. Web nitrogen and hydrogen gas react to form ammonia according to the reaction: Suppose you have 11.0 mol of n, and 9.0.
What volume of ammonia would be produced by this. N2 (g) + 3h2 (g) +2nh3 (g) at a certain temperature and pressure, 1.1 l of n2 reacts with. Asked • 03/19/18 in the reaction of nitrogen gas with hydrogen gas to form ammonia gas, how many grams of hydrogen are needed to produce 1.80. Web nitrogen gas and hydrogen gas react to form ammonia according to the following thermochemical equation. Web this reaction is the synthesis of ammonia using nitrogen and hydrogen gas. Web nitrogen and hydrogen gases react to form ammonia gas via the following reaction: Nitrogen gas (n 2) reacts with hydrogen gas (h 2) to form ammonia (nh 3 ). Calculate the largest amount of. 3h2+n2 2nh3δh=−96kj write the balanced. Web expert answer 86% (35 ratings) transcribed image text:
Nitrogen gas and hydrogen gas react to form ammonia according to the following thermochemical equation. 1 n2 (s) + 3 h2 (g) → 2 nh3 (g)what mass of nitrogen is required to completely react with 800.0 ml. Web chemistry chemistry questions and answers 161 14. If there are 75.0 grams of nitrogen and 45.0. Web nitrogen (n2) gas and hydrogen (h2) gas react to form ammonia (nh3) gas. Nitrogen gas and hydrogen gas react together to form ammonia (nhs). Web expert answer 86% (35 ratings) transcribed image text: Web science chemistry chemistry questions and answers nitrogen (n2) gas and hydrogen (h2) gas react to form ammonia (nh3) gas. Use the chemical equation to complete the statements. Asked • 03/19/18 in the reaction of nitrogen gas with hydrogen gas to form ammonia gas, how many grams of hydrogen are needed to produce 1.80.
Solved Ammonia (NH3) gas and oxygen (02) gas react to form
Web nitrogen gas and hydrogen gas react to form ammonia according to the following thermochemical equation. Web nitrogen and hydrogen gases react to form ammonia gas via the following reaction: Suppose you have 13.0 mol of n2 and 1.0 mol of h2 in a reactor. Hydrogen gas and nitrogen gas react to form ammonia gas. Web expert answer 86% (35.
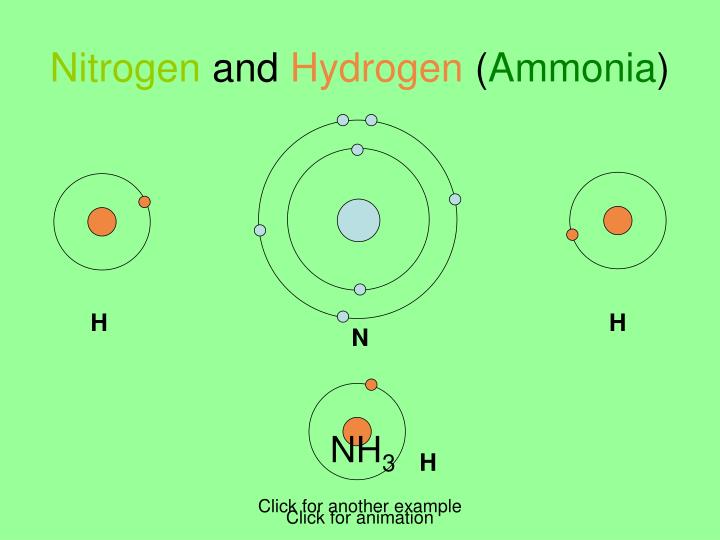
PPT Covalent Bonding PowerPoint Presentation ID5648526
Use the chemical equation to complete the statements. What volume of ammonia would be produced by this reaction if 8.8 m² of nitrogen were consumed? If there are 75.0 grams of nitrogen and 45.0. Calculate the largest amount of. Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as;
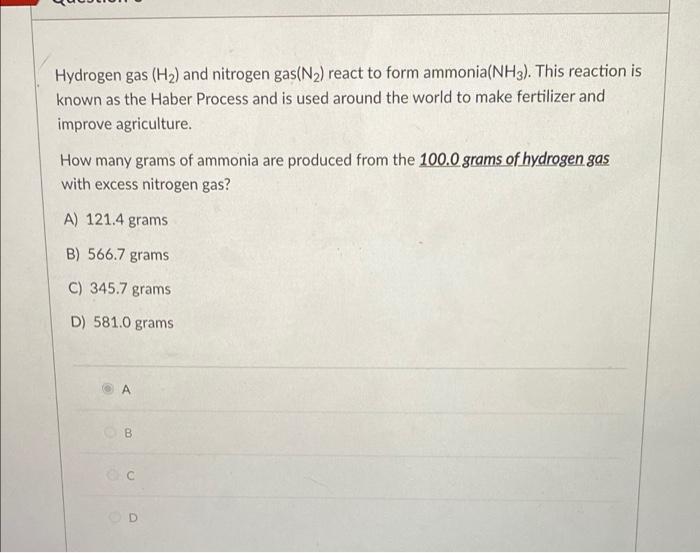
Solved Hydrogen gas (H2) and nitrogen gas(N2) react to form
Asked • 03/19/18 in the reaction of nitrogen gas with hydrogen gas to form ammonia gas, how many grams of hydrogen are needed to produce 1.80. Web hydrogen gas combines with nitrogen to form ammonia. Web nitrogen (n2) gas and hydrogen (h2) gas react to form ammonia (nh3) gas. What volume of ammonia would be produced by this. Nitrogen gas.
Nitrogen gas and hydrogen gas combine to form gaseous ammonia
Web chemistry chemistry questions and answers 161 14. Suppose you have 13.0 mol of n2 and 1.0 mol of h2 in a reactor. Web nitrogen (n2) gas and hydrogen (h2) gas react to form ammonia (nh3) gas. Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as; Web nitrogen.
Solved D Question 9 1 pts Nitrogen and hydrogen gas react to
Calculate the largest amount of. 1 n2 (s) + 3 h2 (g) → 2 nh3 (g)what mass of nitrogen is required to completely react with 800.0 ml. At 200°c in a closed container, 1.0 atm of nitrogen gas is mixed with 2.0 atm of hydrogen gas. Suppose you have 11.0 mol of n, and 9.0. Hydrogen gas and nitrogen gas.
Missing Link for Solar Hydrogen is... Ammonia? SolarPACES
Web nitrogen and hydrogen gases react to form ammonia gas via the following reaction: Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as; At 200°c in a closed container, 1.00 atm of nitrogen gas is mixed with 2.00 atm of. 3h2+n2 2nh3δh=−96kj write the balanced. Web counting atoms.
D Question 9 1 pts Nitrogen and hydrogen gas react to form ammonia (NH3
Calculate the largest amount of. Web nitrogen gas and hydrogen gas react to form ammonia according to the following thermochemical equation. Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as; If there are 75.0 grams of nitrogen and 45.0. 1 n2 (s) + 3 h2 (g) → 2.
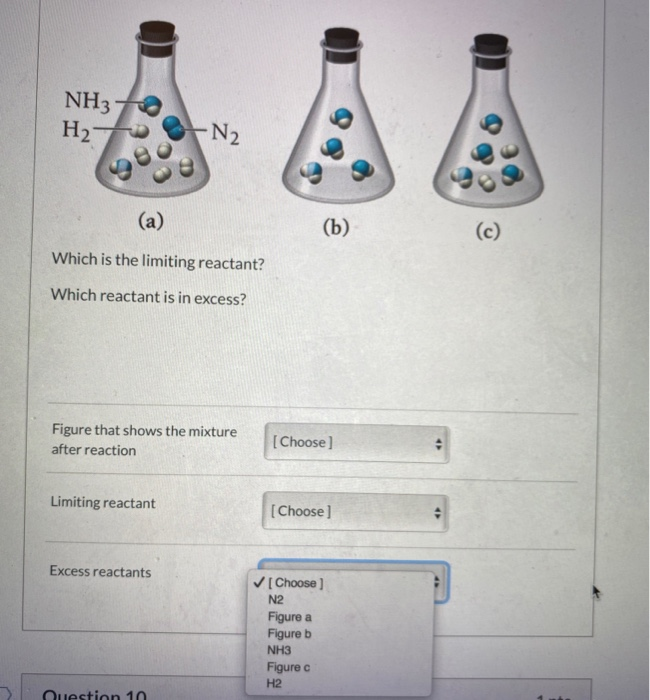
Solved Nitrogen (N2) gas and hydrogen (H2) gas react to form
Web nitrogen and hydrogen gases react to form ammonia gas via the following reaction: Nitrogen gas and hydrogen gas react to form ammonia according to the following thermochemical equation. Nitrogen gas (n 2) reacts with hydrogen gas (h 2) to form ammonia (nh 3 ). Web if a reaction just involves gases, then the coefficients can be used to show.
Solved 10 g of nitrogen is reacted with 5.0 g of hydrogen to
Web expert answer 86% (35 ratings) transcribed image text: Web nitrogen and hydrogen gases react to form ammonia gas via the following reaction: Web nitrogen and hydrogen combine to form ammonia via the following reaction: Web if a reaction just involves gases, then the coefficients can be used to show relationships between moles of reactant and products and also leaders.
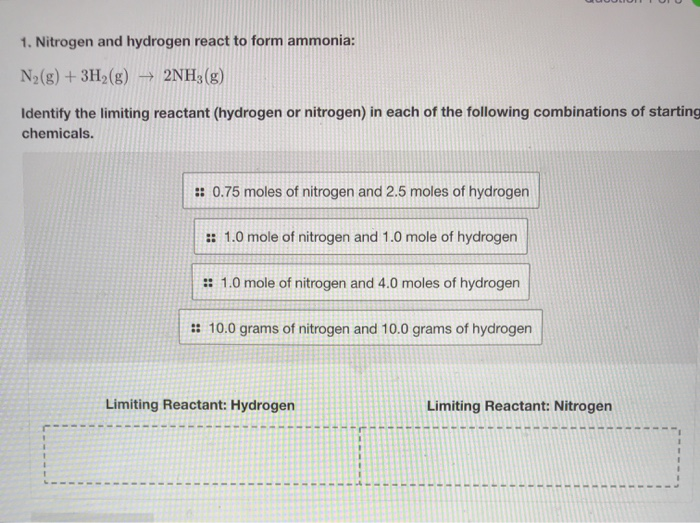
Solved 1. Nitrogen and hydrogen react to form ammonia N2(g)
Web this reaction is the synthesis of ammonia using nitrogen and hydrogen gas. 1 n2 (s) + 3 h2 (g) → 2 nh3 (g)what mass of nitrogen is required to completely react with 800.0 ml. Web expert answer 86% (35 ratings) transcribed image text: What volume of ammonia would be produced by this reaction if 8.8 m² of nitrogen were.
Web The Following Chemical Reaction Takes Place When Hydrogen Gas Reacts With Nitrogen To Form Ammonia Which Can Be Shown As;
Calculate the largest amount of. Web 1 day agofortunately, ammonia is a promising hydrogen carrier that can be easily liquified under milder conditions, transported, and decomposed with the help of a catalyst. What volume of ammonia would be produced by this reaction if 8.8 m² of nitrogen were consumed? Web science chemistry chemistry questions and answers nitrogen (n2) gas and hydrogen (h2) gas react to form ammonia (nh3) gas.
Web Nitrogen (N2) Gas And Hydrogen (H2) Gas React To Form Ammonia (Nh3) Gas.
Web chemistry chemistry questions and answers 161 14. Asked • 03/19/18 in the reaction of nitrogen gas with hydrogen gas to form ammonia gas, how many grams of hydrogen are needed to produce 1.80. Web nitrogen gas (n₂) reacts with hydrogen gas (h₂) to form ammonia (nh₃). Web if a reaction just involves gases, then the coefficients can be used to show relationships between moles of reactant and products and also leaders of reactant and products,.
Web Nitrogen And Hydrogen Gases React To Form Ammonia Gas Via The Following Reaction:
Web nitrogen and hydrogen combine to form ammonia via the following reaction: Web counting atoms in chemical equations nitrogen gas reacts with hydrogen to produce ammonia. Web hydrogen gas and nitrogen gas react to form ammonia gas. What volume of ammonia would be produced by this.
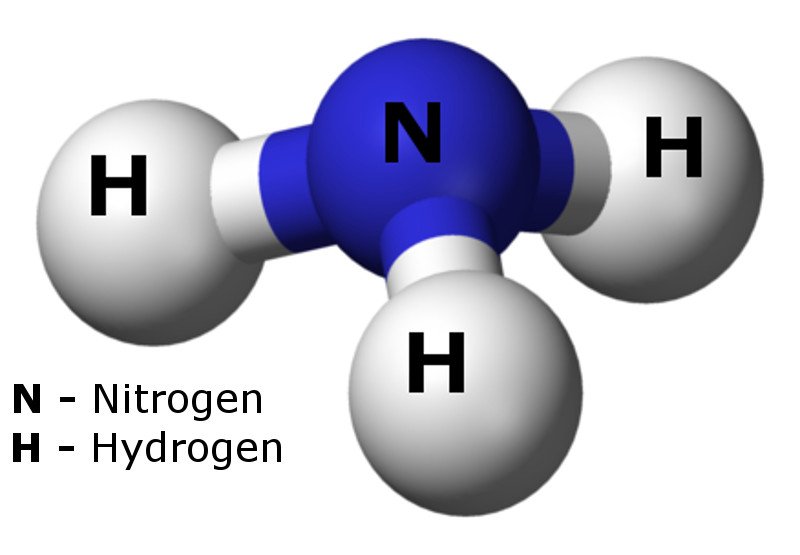
Nitrogen Gas (N 2) Reacts With Hydrogen Gas (H 2) To Form Ammonia (Nh 3 ).
Web nitrogen and hydrogen gas react to form ammonia according to the reaction: H 2 ( g) hydrogen + n 2 ( g) nitrogen ⇌ nh 3 ( g). Use the chemical equation to complete the statements. 1 n2 (s) + 3 h2 (g) → 2 nh3 (g)what mass of nitrogen is required to completely react with 800.0 ml.