Sodium Oxide Combines With Water To Form Sodium Hydroxide
Sodium Oxide Combines With Water To Form Sodium Hydroxide - Na2o + h2o → 2nah + o2 c. Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution. Web generally, elemental sodium is more reactive than lithium, and it reacts with water to form a strong base, sodium hydroxide (naoh). Web sodium oxide + water → sodium hydroxide [na2o + h2o → 2 naoh] is an example of get the answers you need, now! Na2o + h2o → 2nah + o2. Sodium oxide reacts with water to produce sodium hydroxide. Depending on its concentration, this will have a ph around. Web who balanced equation for to reaction between sodium oxide and water is. The reaction is highly exothermic. Web solution reaction of sodium oxide with water:
Na2o + h2o → 2na +. Na2o + h2o → 2nah + o2 c. Web sodium oxide reacts with water to give sodium hydroxide. Web sodium oxide + water → sodium hydroxide [na2o + h2o → 2 naoh] is an example of get the answers you need, now! Na2o + h2o → 2naoh b. Asked • 08/03/15 the balanced equation for the reaction between sodium oxide and water is a. Web who balanced equation for to reaction between sodium oxide and water is. Na2o + h2o → 2naoh b. Reaction with alcohols gives their. Sodium oxide reacts with water to produce sodium hydroxide.
The reaction is highly exothermic. Asked • 08/03/15 the balanced equation for the reaction between sodium oxide and water is a. Sodium oxide reacts with water to produce sodium hydroxide. First of all, the equation. How many grams of sodium. The balanced chemical equation for sodium oxide. Web solution reaction of sodium oxide with water: Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution. Na2o + h2o → 2naoh b. Web expert answer transcribed image text:
Chemical Equation Between Sodium Oxide And Water Tessshebaylo
The reaction is highly exothermic. Web generally, elemental sodium is more reactive than lithium, and it reacts with water to form a strong base, sodium hydroxide (naoh). Depending on its concentration, this will have a ph around. Na+h2o>naoh that is sodium reacting with water to give sodium hydroxide. Web can you please balance this equation for me:
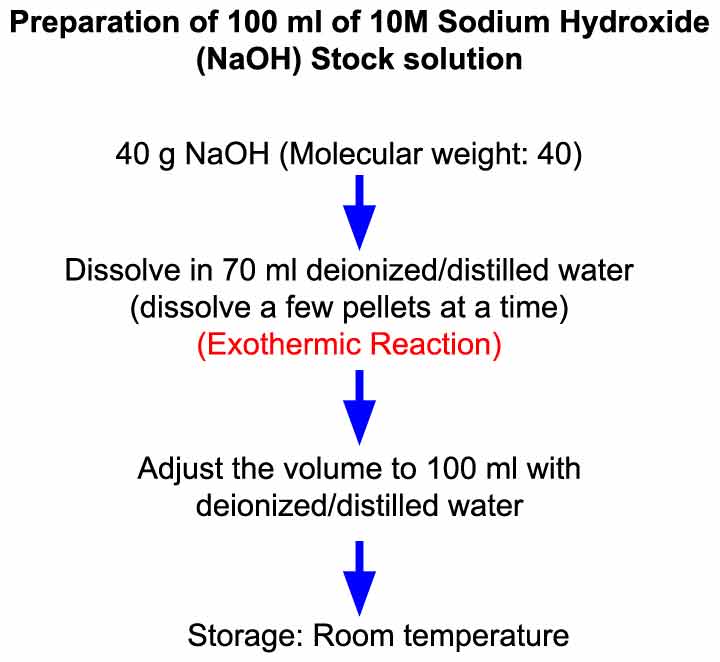
Preparation of 10 M Sodium Hydroxide (NaOH) Solution Laboratory Notes
Web solution reaction of sodium oxide with water: Web sodium oxide reacts with water to give sodium hydroxide. Web yes it is a hydroxide for a word equasion it is sodium + water = sodium hydroxide + hydrogen what is sodium oxide combines with water to make sodium. Web science chemistry chemistry questions and answers write the word equation and.
Pure Sodium Hydroxide Powder, Packing Size 50 Kg, Rs 105 /kilogram
Web who balanced equation for to reaction between sodium oxide and water is. Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution. The reaction is highly exothermic. Web science chemistry chemistry questions and answers write the word equation and a balanced chemical equation for each reaction below. Its chemistry is well explored.
Sodium hydroxide, 1.000M, 2.5L Vintessential Wine Laboratories
Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution. Na2o + h2o → 2naoh b. Web generally, elemental sodium is more reactive than lithium, and it reacts with water to form a strong base, sodium hydroxide (naoh). Asked • 08/03/15 the balanced equation for the reaction between sodium oxide and water is a. Web can you please.
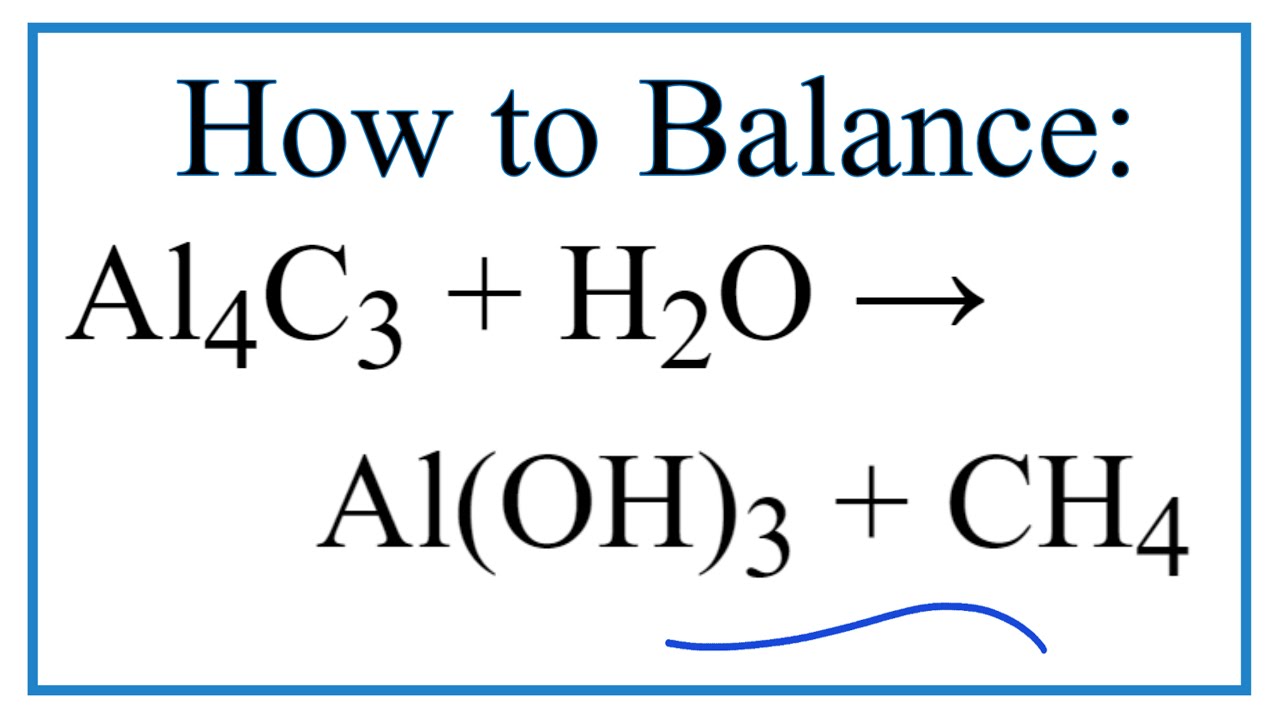
How to Balance Al4C3 + H2O = Al(OH)3 + CH4 (Aluminum carbide + Water
Web sodium oxide reacts with water to give sodium hydroxide. Na2o + h2o → 2naoh b. Web science chemistry chemistry questions and answers write the word equation and a balanced chemical equation for each reaction below. Its chemistry is well explored. Web expert answer transcribed image text:
SODIUM HYDROXIDE, 1.0N 500ML H
Its chemistry is well explored. Sodium oxide combines with water to form sodium hydroxide. Web who balanced equation for to reaction between sodium oxide and water is. Web can you please balance this equation for me: Na+h2o>naoh that is sodium reacting with water to give sodium hydroxide.
Solved Nitrogen plus Hydrogen produce Nitrogen Trihydride.
Web sodium oxide + water → sodium hydroxide [na2o + h2o → 2 naoh] is an example of get the answers you need, now! Web solution reaction of sodium oxide with water: Na2o + h2o → 2nah + o2. Its chemistry is well explored. Na 2 o + h 2 o → 2 naoh.
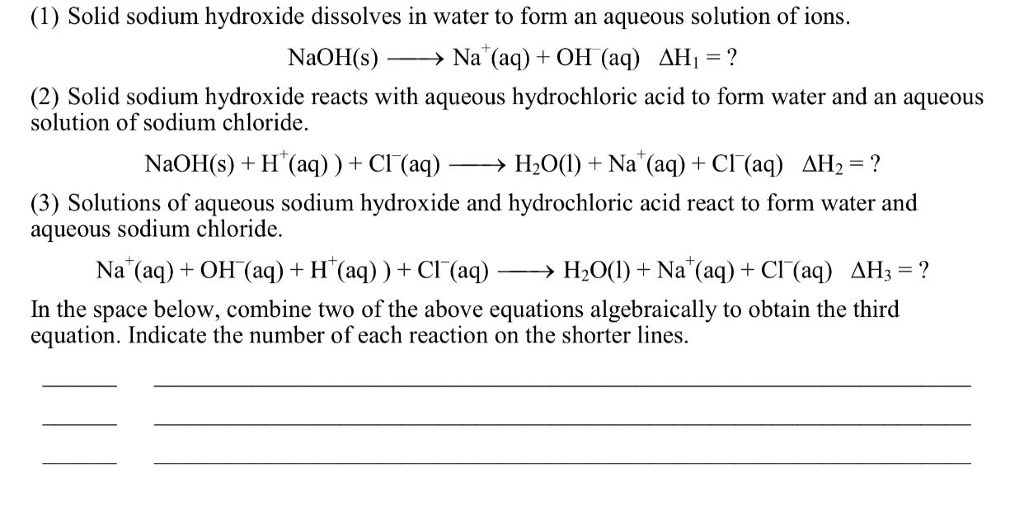
Solved (1) Solid sodium hydroxide dissolves in water to form
Na2o + h2o → 2naoh b. Web can you please balance this equation for me: Web yes it is a hydroxide for a word equasion it is sodium + water = sodium hydroxide + hydrogen what is sodium oxide combines with water to make sodium. Web sodium oxide + water → sodium hydroxide [na2o + h2o → 2 naoh] is.
Sodium Hydroxide, Grade Standard Technical Grade, Rs 25.20 /kg ID
Web can you please balance this equation for me: Web science chemistry chemistry questions and answers write the word equation and a balanced chemical equation for each reaction below. Web sodium oxide reacts with water to give sodium hydroxide. Na2o + h2o → 2naoh b. Web yes it is a hydroxide for a word equasion it is sodium + water.
Sodium Oxide Combines With Water To Form Sodium Hydroxide.
Asked • 08/03/15 the balanced equation for the reaction between sodium oxide and water is a. Reaction with alcohols gives their. Web sodium oxide + water → sodium hydroxide [na2o + h2o → 2 naoh] is an example of get the answers you need, now! Na2o + h2o → 2naoh b.
Web Yes It Is A Hydroxide For A Word Equasion It Is Sodium + Water = Sodium Hydroxide + Hydrogen What Is Sodium Oxide Combines With Water To Make Sodium.
Na2o + h2o → 2nah + o2 c. First of all, the equation. Na2o + h2o → 2na +. Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution.
The Balanced Chemical Equation For Sodium Oxide.
Web sodium oxide reacts with water to give sodium hydroxide. Na 2 o + h 2 o → 2 naoh. Na+h2o>naoh that is sodium reacting with water to give sodium hydroxide. Web science chemistry chemistry questions and answers write the word equation and a balanced chemical equation for each reaction below.
Na2O + H2O → 2Nah + O2.
Sodium oxide reacts with water to produce sodium hydroxide. Depending on its concentration, this will have a ph around. The reaction is highly exothermic. Na2o + h2o → 2naoh b.