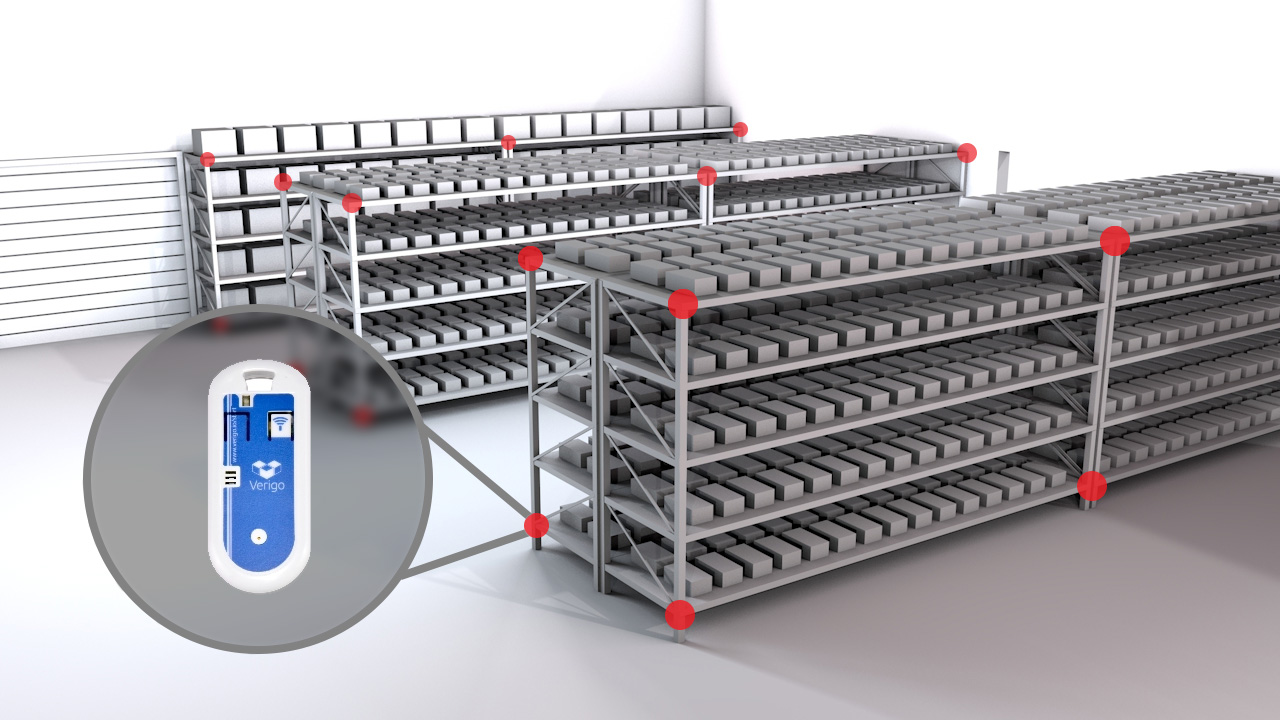
Temperature Mapping Protocol Template
Temperature Mapping Protocol Template - Approval page and change control history Whilst this guide is not exhaustive, it will give you an idea of the questions you should be asking yourself when carrying out a. Xxxxxxxx] dfasdf dfasdf you can buy a full version of this template in ms word format that shall be completely editable, ready to use according to your needs, visiting: • specify the management approval process, especially for adverse events such as temperature deviations. Planning, including determining optimal sensor type and placement, and how the. Web the mapping protocol should contain the following sections: Web thermal mapping protocol template [protocol number: Web 2.3 qualification protocols 15 2.3.1 approval page and change control history 15. Web detailed procedure and guidelines on temperature mapping study & qualification of cold rooms, warehouses, vans, trucks, reefers, refrigerators & boxes for pharmaceutical industry temperature mapping study and temperature qualification study are carried out generally in. Annexes as needed, including templates for the mapping report.
Temperature mapping establishes the temperature distribution within the zonebeing mapped and it locates hot and cold spots. Web 2.3 qualification protocols 15 2.3.1 approval page and change control history 15. Web the mapping protocol should contain the following sections: • create change control protocols so it’s clear when changes such as maintenance, new construction, and reconfiguration of racks will require revalidation. Web temperature mapping protocols will vary between the individuals unique requirements. Approval page and change control history Web a temperature or humidity excursion occurs. 2.3.7 qualification report template 17 2.3.8 approval process 18 2.4 installation qualification 18 2.4.1 identifying critical components 19 2.4.2 checking installed systems, subsystems and components 19. Web 2.2 the mapping protocol 11 2.2.1 approval page and change control history 11 2.2.2 acronyms and glossary 12 2.2.3 description and rationale 12 2.2.4 scope 12 2.2.5 objectives 13 2.2.6 methodology 14 2.2.7 mapping report template 18 2.3 conducting the mapping exercise 18 2.4 analysing the data and preparing the mapping report 18 Temperature mapping of storage areas.
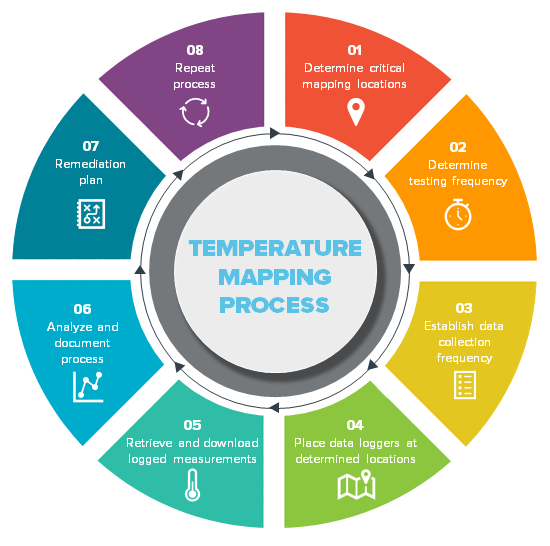
It can be broken down into three phases: Web detailed procedure and guidelines on temperature mapping study & qualification of cold rooms, warehouses, vans, trucks, reefers, refrigerators & boxes for pharmaceutical industry temperature mapping study and temperature qualification study are carried out generally in. Web 2.3 qualification protocols 15 2.3.1 approval page and change control history 15. Approval page and change control history b. • specify the management approval process, especially for adverse events such as temperature deviations. If you have questions about cold storage mapping, please contact your thermo fisher sales or service representative. This information should serve as a starting point as you implement your temperature mapping process and reinforce some of the basics for those who do it every day. Web understand what cold storage temperature mapping entails. Web the mapping protocol should contain the following sections: Whilst this guide is not exhaustive, it will give you an idea of the questions you should be asking yourself when carrying out a.
Apoteker Oke Temperature Mapping
Approval page and change control history b. It can be broken down into three phases: Whilst this guide is not exhaustive, it will give you an idea of the questions you should be asking yourself when carrying out a. Web 2.3 qualification protocols 15 2.3.1 approval page and change control history 15. • create change control protocols so it’s clear.
Temperature Mapping Services IntroTech, the Cold Chain specialist
Web 2.3 qualification protocols 15 2.3.1 approval page and change control history 15. Web the mapping protocol should contain the following sections: Web understand what cold storage temperature mapping entails. Web temperature mapping protocols will vary between the individuals unique requirements. However, the process does not need to be complicated.
Temperature Mapping Validation Services Anova
• specify the management approval process, especially for adverse events such as temperature deviations. 2.3.7 qualification report template 17 2.3.8 approval process 18 2.4 installation qualification 18 2.4.1 identifying critical components 19 2.4.2 checking installed systems, subsystems and components 19. Annexes as needed, including templates for the mapping report. Web detailed procedure and guidelines on temperature mapping study & qualification.
Temperature Mapping Qualification Protocol for Refrigerator
It can be broken down into three phases: 2.3.7 qualification report template 17 2.3.8 approval process 18 2.4 installation qualification 18 2.4.1 identifying critical components 19 2.4.2 checking installed systems, subsystems and components 19. However, the process does not need to be complicated. Web the mapping protocol should contain the following sections: Annexes as needed, including templates for the mapping.
Temperature mapping study & qualification protocol procedure
Web understand what cold storage temperature mapping entails. • specify the management approval process, especially for adverse events such as temperature deviations. • determine a validation schedule. Web detailed procedure and guidelines on temperature mapping study & qualification of cold rooms, warehouses, vans, trucks, reefers, refrigerators & boxes for pharmaceutical industry temperature mapping study and temperature qualification study are carried.
Warehouse Temperature Mapping Protocol map Resume Examples Wk9y6EE0Y3
• create change control protocols so it’s clear when changes such as maintenance, new construction, and reconfiguration of racks will require revalidation. Xxxxxxxx] dfasdf dfasdf you can buy a full version of this template in ms word format that shall be completely editable, ready to use according to your needs, visiting: Approval page and change control history Web 2.2 the.
Temperature Mapping 7 Day Labcold
Web temperature mapping protocols will vary between the individuals unique requirements. It can be broken down into three phases: Xxxxxxxx] dfasdf dfasdf you can buy a full version of this template in ms word format that shall be completely editable, ready to use according to your needs, visiting: Web 2.2 the mapping protocol 11 2.2.1 approval page and change control.
temperaturemappingstudyprotocolprocedure.pdf Refrigeration
It can be broken down into three phases: Web 2.3 qualification protocols 15 2.3.1 approval page and change control history 15. A temperature mapping exercise is required for any space allocated for the storage and handling of products with a specified labelled storage temperature. Planning, including determining optimal sensor type and placement, and how the. Temperature mapping of storage areas.
WHO Guidelines Temperature mapping of storage areas free PDF
Annexes as needed, including templates for the mapping report. Web temperature mapping protocols will vary between the individuals unique requirements. In this month’s blog, we look at how to do temperature mapping. Web understand what cold storage temperature mapping entails. Xxxxxxxx] dfasdf dfasdf you can buy a full version of this template in ms word format that shall be completely.
Temperature Mapping of Storage Areas for Pharmaceuticals Incepbio
Xxxxxxxx] dfasdf dfasdf you can buy a full version of this template in ms word format that shall be completely editable, ready to use according to your needs, visiting: Web the mapping protocol should contain the following sections: Planning, including determining optimal sensor type and placement, and how the. It can be broken down into three phases: Web thermal mapping.
However, The Process Does Not Need To Be Complicated.
Approval page and change control history b. Web 2.2 the mapping protocol 11 2.2.1 approval page and change control history 11 2.2.2 acronyms and glossary 12 2.2.3 description and rationale 12 2.2.4 scope 12 2.2.5 objectives 13 2.2.6 methodology 14 2.2.7 mapping report template 18 2.3 conducting the mapping exercise 18 2.4 analysing the data and preparing the mapping report 18 Planning, including determining optimal sensor type and placement, and how the. It can be broken down into three phases:
Xxxxxxxx] Dfasdf Dfasdf You Can Buy A Full Version Of This Template In Ms Word Format That Shall Be Completely Editable, Ready To Use According To Your Needs, Visiting:
Web 2.3 qualification protocols 15 2.3.1 approval page and change control history 15. In this month’s blog, we look at how to do temperature mapping. If you have questions about cold storage mapping, please contact your thermo fisher sales or service representative. Web understand what cold storage temperature mapping entails.
• Create Change Control Protocols So It’s Clear When Changes Such As Maintenance, New Construction, And Reconfiguration Of Racks Will Require Revalidation.
This information should serve as a starting point as you implement your temperature mapping process and reinforce some of the basics for those who do it every day. Web detailed procedure and guidelines on temperature mapping study & qualification of cold rooms, warehouses, vans, trucks, reefers, refrigerators & boxes for pharmaceutical industry temperature mapping study and temperature qualification study are carried out generally in. Web the mapping protocol should contain the following sections: Temperature mapping establishes the temperature distribution within the zonebeing mapped and it locates hot and cold spots.
Whilst This Guide Is Not Exhaustive, It Will Give You An Idea Of The Questions You Should Be Asking Yourself When Carrying Out A.
Web a temperature or humidity excursion occurs. Temperature mapping of storage areas. 2.3.7 qualification report template 17 2.3.8 approval process 18 2.4 installation qualification 18 2.4.1 identifying critical components 19 2.4.2 checking installed systems, subsystems and components 19. • determine a validation schedule.