What Type Of Ions Do Metals Form
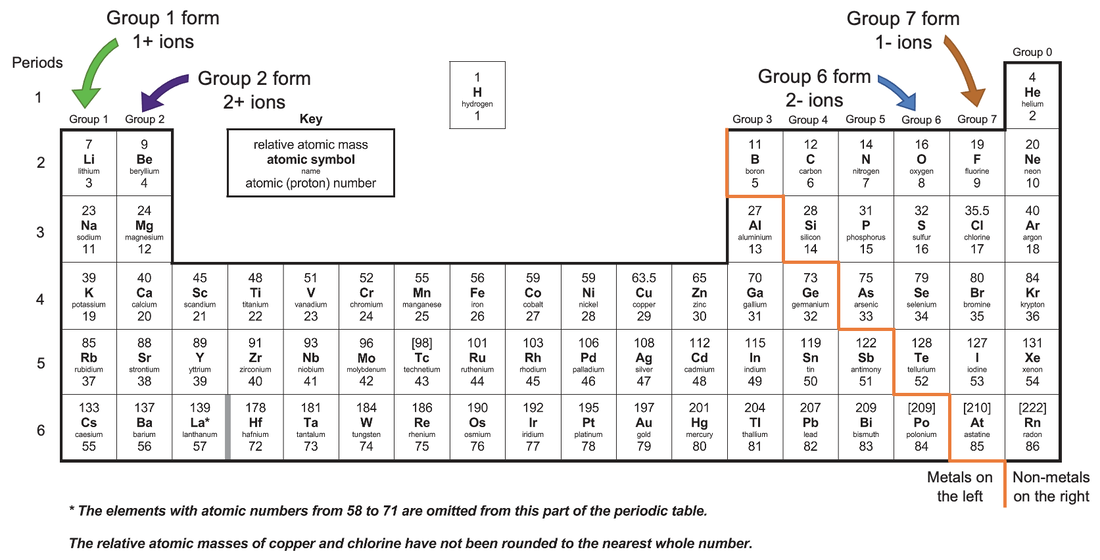
What Type Of Ions Do Metals Form - In these compounds you have the cation, often an alkali metal itself, stabilized in. By combination of ions with other particles; Web metal ions in soil are rarely found in the free state, but mostly occur in interactions with other soil organic, inorganic, and biological components, in the liquid and solid phases. Web what type of ions do metals naturally form? They are all metals and include many common metals. Web metals cations non metals usually form what type of ions? Web 10 transition metals the transition metals are in the block in the middle of the periodic table, between groups 2 and 3. According to the spdf theory, metals can have either a fixed number of electrons that they can lose, or can have multiple numbers of electrons they. It consists of potassium (k+) ions (group 1 metals always form 1+ ions) and nitrate. Web metal compounds tend to be ionic as metal atoms readily form positive ions.
Web the chemical differences between metals and nonmetals that interest us the most: Web ions are formed by the addition of electrons to, or the removal of electrons from, neutral atoms or molecules or other ions; Charges of +2 or +3 are common, but charges ranging from +1. Transition metal ions are essential cofactors for proteins with diverse functions, including electron transfer, dioxygen binding and activation, nitrogen fixation,. Web positively charged ions are called cations. Web first, metals tend to from cations and nonmetals tend to form anions, while the noble gasses do not tend to form ions. Web 10 transition metals the transition metals are in the block in the middle of the periodic table, between groups 2 and 3. Web metals cations non metals usually form what type of ions? Ions form when atoms lose or gain electrons to obtain a full outer shell: By combination of ions with other particles;
Web ions are formed by the addition of electrons to, or the removal of electrons from, neutral atoms or molecules or other ions; It consists of potassium (k+) ions (group 1 metals always form 1+ ions) and nitrate. Web first, metals tend to from cations and nonmetals tend to form anions, while the noble gasses do not tend to form ions. Some atoms have nearly eight electrons in their. A nonmetal usually form an anion how do ions in metals behave? They are all metals and include many common metals. According to the spdf theory, metals can have either a fixed number of electrons that they can lose, or can have multiple numbers of electrons they. Transition metal ions are essential cofactors for proteins with diverse functions, including electron transfer, dioxygen binding and activation, nitrogen fixation,. Web metal compounds tend to be ionic as metal atoms readily form positive ions. Web forming ions an ion is an atom or group of atoms with a positive or negative charge.
Chem matters ch6_ionic_bond
Web what type of ions do metals naturally form? Second, the nonmetals have only one. Some atoms have nearly eight electrons in their. Web ions are formed by the addition of electrons to, or the removal of electrons from, neutral atoms or molecules or other ions; According to the spdf theory, metals can have either a fixed number of electrons.
Periodic Table Which Groups Of Elements Tend To Form Positive Ions
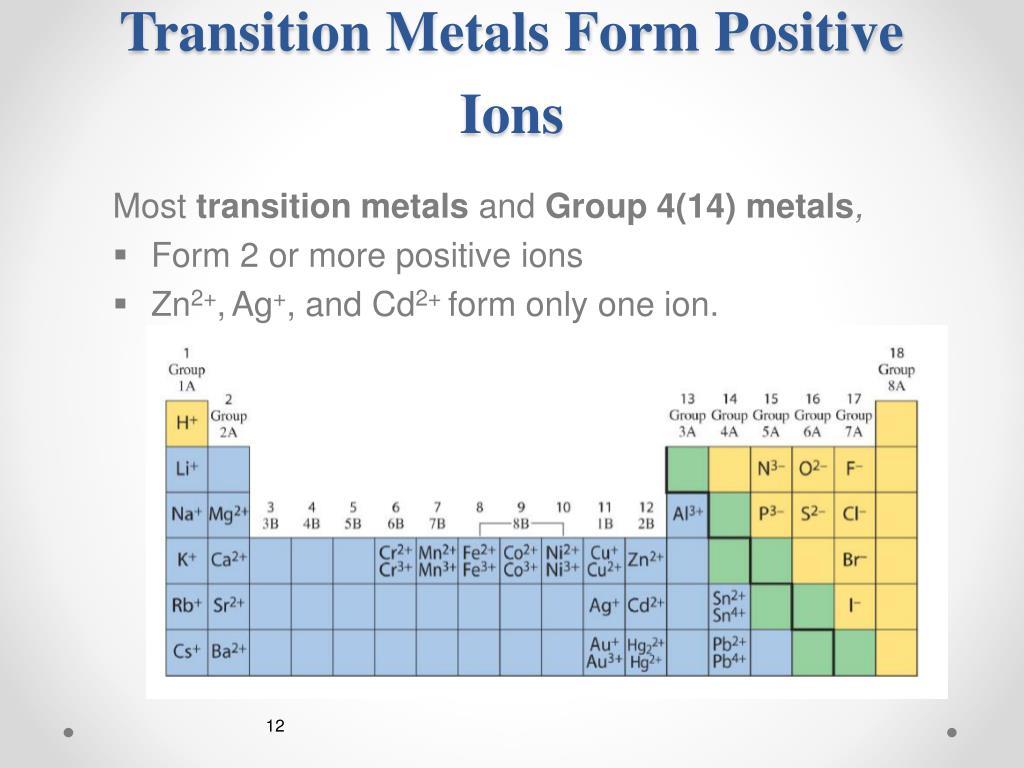
These elements form cations with varying extents of charge. As you also have heard them as transition metals. Web positively charged ions are called cations. Web the chemical differences between metals and nonmetals that interest us the most: Web 10 transition metals the transition metals are in the block in the middle of the periodic table, between groups 2 and.
Ionic Bond Definition, Types, Properties & Examples
Transition metal ions are essential cofactors for proteins with diverse functions, including electron transfer, dioxygen binding and activation, nitrogen fixation,. Web chemistry matter elements 1 answer umair.a jul 3, 2016 they form cations (positively charged ion). Ions form when atoms lose or gain electrons to obtain a full outer shell: Web metal ions in soil are rarely found in the.
CH103 CHAPTER 4 Ions and Ionic Compounds Chemistry
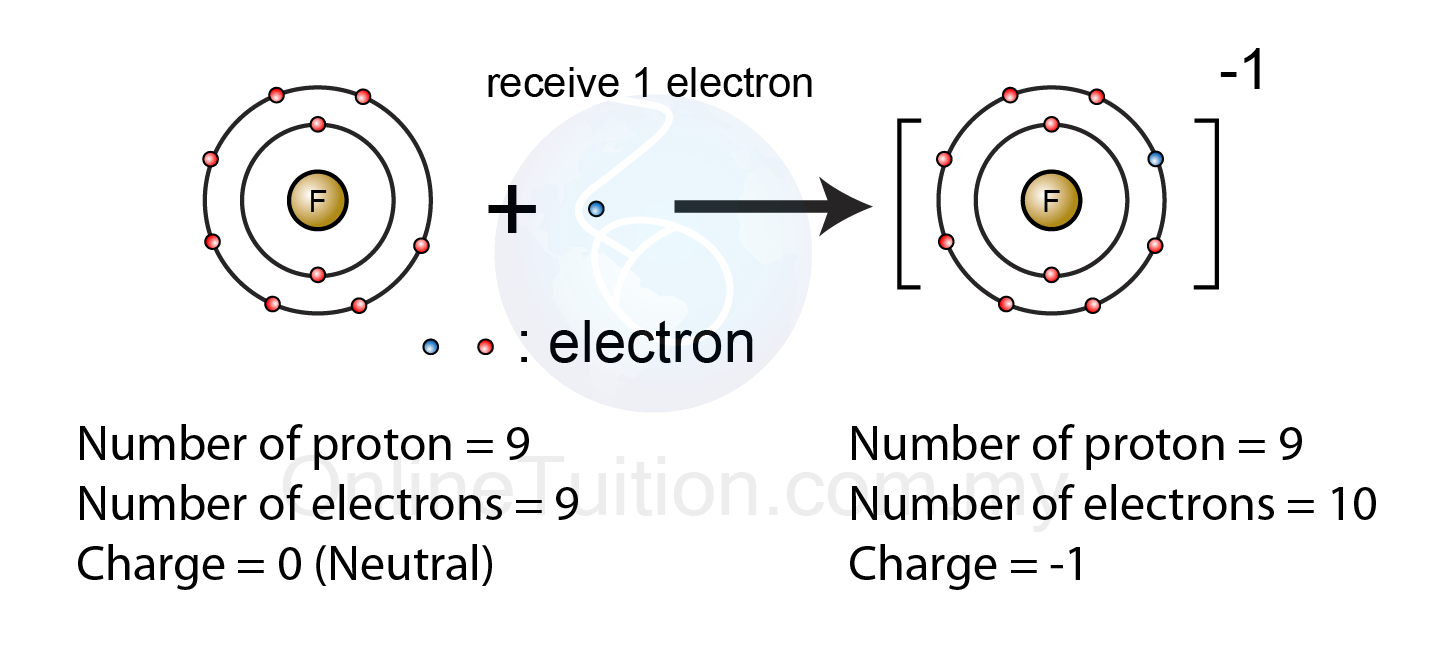
In these compounds you have the cation, often an alkali metal itself, stabilized in. Most metals become cations when they make ionic compounds. As you also have heard them as transition metals. (3 points) negative ions, by gaining electrons to fill the valence shell negative ions, by losing electrons to empty the valence shell. Some atoms have nearly eight electrons.
PPT Chapter 4 Compounds and Their Bonds PowerPoint Presentation, free
Web 10 transition metals the transition metals are in the block in the middle of the periodic table, between groups 2 and 3. Web ions are formed by the addition of electrons to, or the removal of electrons from, neutral atoms or molecules or other ions; Transition metal ions are essential cofactors for proteins with diverse functions, including electron transfer,.
5.2.1 Formation of Ion Revision.my
Web ions are formed by the addition of electrons to, or the removal of electrons from, neutral atoms or molecules or other ions; These elements form cations with varying extents of charge. Web metals cations non metals usually form what type of ions? Web metal compounds tend to be ionic as metal atoms readily form positive ions. (3 points) negative.
Chem matters ch6_ionic_bond
Most metals become cations when they make ionic compounds. Web 10 transition metals the transition metals are in the block in the middle of the periodic table, between groups 2 and 3. They are all metals and include many common metals. Ions form when atoms lose or gain electrons to obtain a full outer shell: It consists of potassium (k+).
C2 B) Ions from the Periodic Table AQA Combined Science Trilogy Elevise
As you also have heard them as transition metals. In these compounds you have the cation, often an alkali metal itself, stabilized in. Ions form when atoms lose or gain electrons to obtain a full outer shell: Web metals cations non metals usually form what type of ions? Web the chemical differences between metals and nonmetals that interest us the.
PPT Naming Ionic Compounds PowerPoint Presentation, free download
These elements form cations with varying extents of charge. Web metal compounds tend to be ionic as metal atoms readily form positive ions. By combination of ions with other particles; Some atoms have nearly eight electrons in their. Transition metal ions are essential cofactors for proteins with diverse functions, including electron transfer, dioxygen binding and activation, nitrogen fixation,.
Naming Simple Ionic Compounds Pathways to Chemistry
A nonmetal usually form an anion how do ions in metals behave? These elements form cations with varying extents of charge. Web the chemical differences between metals and nonmetals that interest us the most: Web metal compounds tend to be ionic as metal atoms readily form positive ions. In these compounds you have the cation, often an alkali metal itself,.
(3 Points) Negative Ions, By Gaining Electrons To Fill The Valence Shell Negative Ions, By Losing Electrons To Empty The Valence Shell.
Web ions are formed by the addition of electrons to, or the removal of electrons from, neutral atoms or molecules or other ions; Web metals cations non metals usually form what type of ions? Web positively charged ions are called cations. Web metal ions in soil are rarely found in the free state, but mostly occur in interactions with other soil organic, inorganic, and biological components, in the liquid and solid phases.
Transition Metal Ions Are Essential Cofactors For Proteins With Diverse Functions, Including Electron Transfer, Dioxygen Binding And Activation, Nitrogen Fixation,.
Web metal compounds tend to be ionic as metal atoms readily form positive ions. These elements form cations with varying extents of charge. Some atoms have nearly eight electrons in their. A nonmetal usually form an anion how do ions in metals behave?
By Combination Of Ions With Other Particles;
Most metals become cations when they make ionic compounds. According to the spdf theory, metals can have either a fixed number of electrons that they can lose, or can have multiple numbers of electrons they. It consists of potassium (k+) ions (group 1 metals always form 1+ ions) and nitrate. Web forming ions an ion is an atom or group of atoms with a positive or negative charge.
In These Compounds You Have The Cation, Often An Alkali Metal Itself, Stabilized In.
They are all metals and include many common metals. Second, the nonmetals have only one. Web first, metals tend to from cations and nonmetals tend to form anions, while the noble gasses do not tend to form ions. Web 10 transition metals the transition metals are in the block in the middle of the periodic table, between groups 2 and 3.