Why Do Atoms Form Bond
Why Do Atoms Form Bond - There are several types of bond. So, in order to fill. Chemical bonds are formed when electrons in different atoms. Web when atoms combine by forming covalent bonds, the resulting collection of atoms is called a molecule. The combination of multiple atoms, or chemical bonding, forms molecules. Attractive forces between atoms that are strong enough to make the linked elements function as a single unit. Web atoms are individual units made up of protons, neutrons, and electrons. Atoms with a full valence electron orbital are less reactive. Web more than one bond can be formed between atoms leading to double and triple bonds. In fact, they have to collide (bump together).
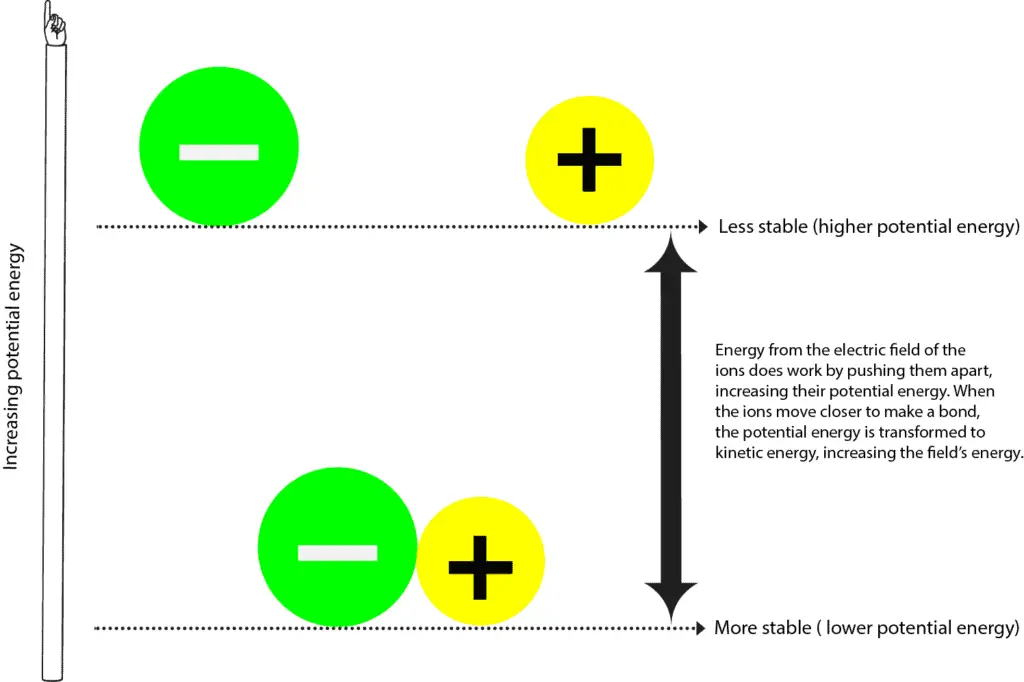
These clusters of strongly bonded atoms are called molecules. Web when atoms combine by forming covalent bonds, the resulting collection of atoms is called a molecule. Scishow explains what makes atoms bond (and what makes them sometimes seem promiscuous). Web atoms have to be close together to form a bond. So, in order to fill. Web why do atoms form chemical bonds? Chemical bonds are formed when electrons in different atoms. Atoms with a full valence electron orbital are less reactive. See how they're represented by. Web the summary of the answer is that the amount of energy involved in the electromagnetic field of two isolated hydrogen atoms is a lot more than the energy.
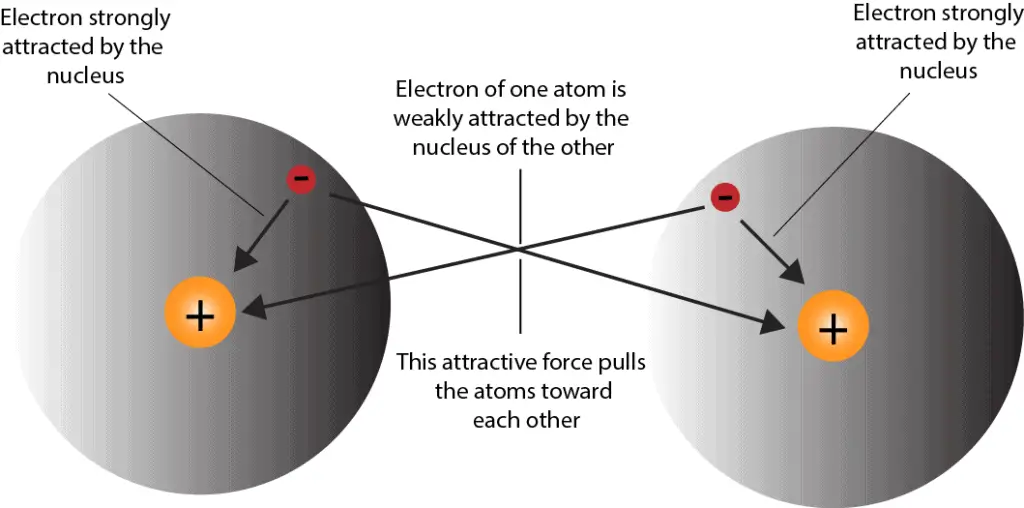
The combination of multiple atoms, or chemical bonding, forms molecules. Scishow explains what makes atoms bond (and what makes them sometimes seem promiscuous). Web when atoms of two or more elements bond together, they make a compound. We can therefore say that a molecule is the simplest unit of a covalent. Web atoms can join together by forming a chemical bond, which is a very strong attraction between two atoms. Web atoms have to be close together to form a bond. Web why do atoms form chemical bonds? In fact, they have to collide (bump together). Atoms with a full valence electron orbital are less reactive. Attractive forces between atoms that are strong enough to make the linked elements function as a single unit.
PPT Ions PowerPoint Presentation, free download ID6738771
The combination of multiple atoms, or chemical bonding, forms molecules. Attractive forces between atoms that are strong enough to make the linked elements function as a single unit. Click the card to flip 👆 atoms form chemical bonds because they want a lower energy and more stable electron configuration. Scishow explains what makes atoms bond (and what makes them sometimes.
Class 9 Chemistry Chapter 4 Lecture 8 Introduction Why do atoms form
Web when atoms combine by forming covalent bonds, the resulting collection of atoms is called a molecule. Chemical bonds are formed when electrons in different atoms. Examples of these are diatomic oxygen (double bond) or nitrogen (triple bond). These clusters of strongly bonded atoms are called molecules. Web when atoms of two or more elements bond together, they make a.
Why Do Atoms Bond? YouTube
Some of the attractive forces are. Attractive forces between atoms that are strong enough to make the linked elements function as a single unit. In fact, they have to collide (bump together). Web atoms are individual units made up of protons, neutrons, and electrons. These clusters of strongly bonded atoms are called molecules.
PPT What are bonds? PowerPoint Presentation, free download ID5980343
Some of the attractive forces are. See how they're represented by. Web why do atoms form chemical bonds? There are several types of bond. With the exception of the noble gases, the other elements do not have completely filled valence shells (the outermost energy shell).
PPT Why do atoms bond? PowerPoint Presentation, free download ID
Web atoms form bonds to try to achieve the same electron configuration as the noble gases. So, in order to fill. Scishow explains what makes atoms bond (and what makes them sometimes seem promiscuous). Web atoms are individual units made up of protons, neutrons, and electrons. The more “crowded” a given space is with atoms, the more likely it is.
Why do atoms bond with one another?
Some of the attractive forces are. So, in order to fill. Web the summary of the answer is that the amount of energy involved in the electromagnetic field of two isolated hydrogen atoms is a lot more than the energy. In fact, they have to collide (bump together). Web atoms can join together by forming a chemical bond, which is.
How do atoms form covalent bond?
So, in order to fill. Chemical bonds are formed when electrons in different atoms. Atoms with a full valence electron orbital are less reactive. Web atoms have to be close together to form a bond. Examples of these are diatomic oxygen (double bond) or nitrogen (triple bond).
Why do atoms bond YouTube
The more “crowded” a given space is with atoms, the more likely it is that. Web atoms form bonds to try to achieve the same electron configuration as the noble gases. Click the card to flip 👆 atoms form chemical bonds because they want a lower energy and more stable electron configuration. Web why do atoms form chemical bonds? Attractive.
Why Do Atoms Form Chemical Bond in Urdu Hindi Lecture /Chemistry for
Web atoms have to be close together to form a bond. Atoms with a full valence electron orbital are less reactive. These clusters of strongly bonded atoms are called molecules. Web atoms can join together by forming a chemical bond, which is a very strong attraction between two atoms. There are several types of bond.
Why do atoms form bonds? YouTube
Web atoms are individual units made up of protons, neutrons, and electrons. Scishow explains what makes atoms bond (and what makes them sometimes seem promiscuous). Web atoms have to be close together to form a bond. There are several types of bond. Atoms with a full valence electron orbital are less reactive.
The More “Crowded” A Given Space Is With Atoms, The More Likely It Is That.
With the exception of the noble gases, the other elements do not have completely filled valence shells (the outermost energy shell). We can therefore say that a molecule is the simplest unit of a covalent. Web more than one bond can be formed between atoms leading to double and triple bonds. Examples of these are diatomic oxygen (double bond) or nitrogen (triple bond).
See How They're Represented By.
In fact, they have to collide (bump together). Some of the attractive forces are. Web atoms can join together by forming a chemical bond, which is a very strong attraction between two atoms. The combination of multiple atoms, or chemical bonding, forms molecules.
These Clusters Of Strongly Bonded Atoms Are Called Molecules.
Web atoms form bonds to try to achieve the same electron configuration as the noble gases. Web atoms are individual units made up of protons, neutrons, and electrons. Web atoms have to be close together to form a bond. Atoms with a full valence electron orbital are less reactive.
So, In Order To Fill.
Chemical bonds are formed when electrons in different atoms. Web the summary of the answer is that the amount of energy involved in the electromagnetic field of two isolated hydrogen atoms is a lot more than the energy. Web when atoms of two or more elements bond together, they make a compound. Click the card to flip 👆 atoms form chemical bonds because they want a lower energy and more stable electron configuration.