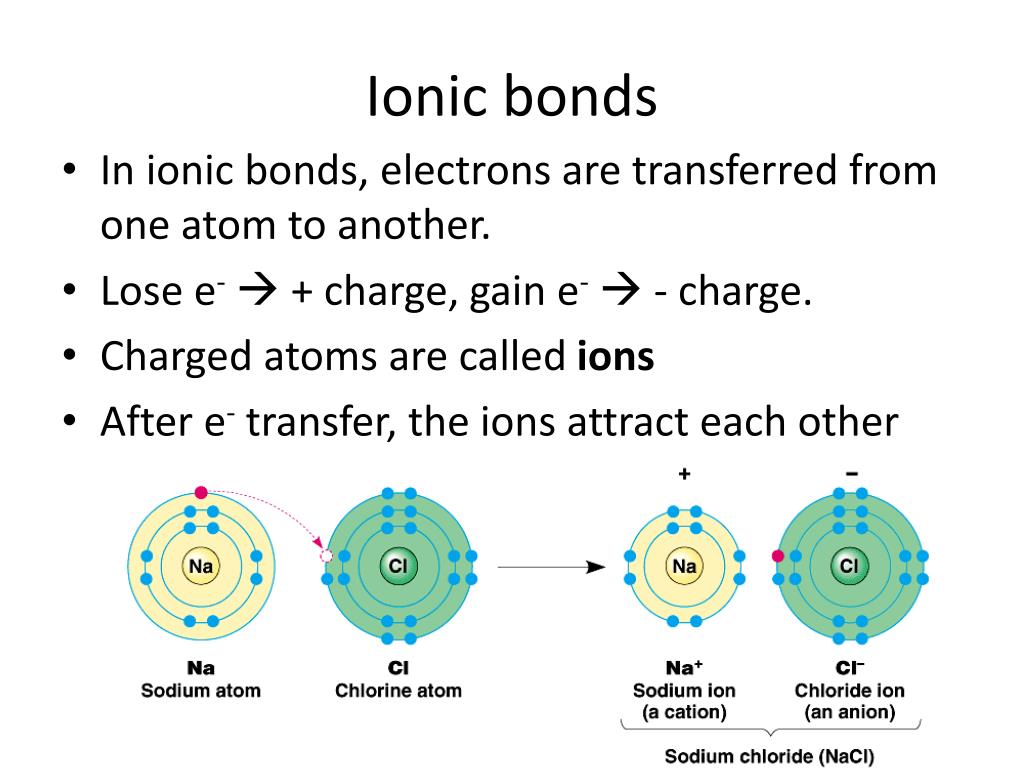
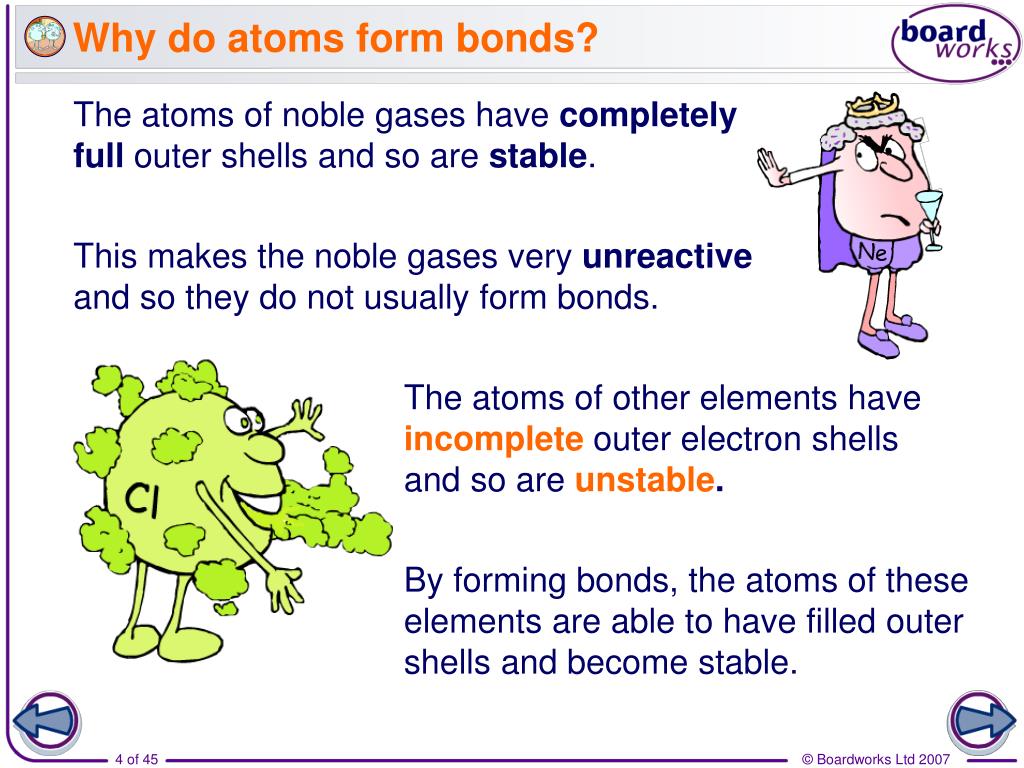
Why Do Atoms Form Chemical Bonds
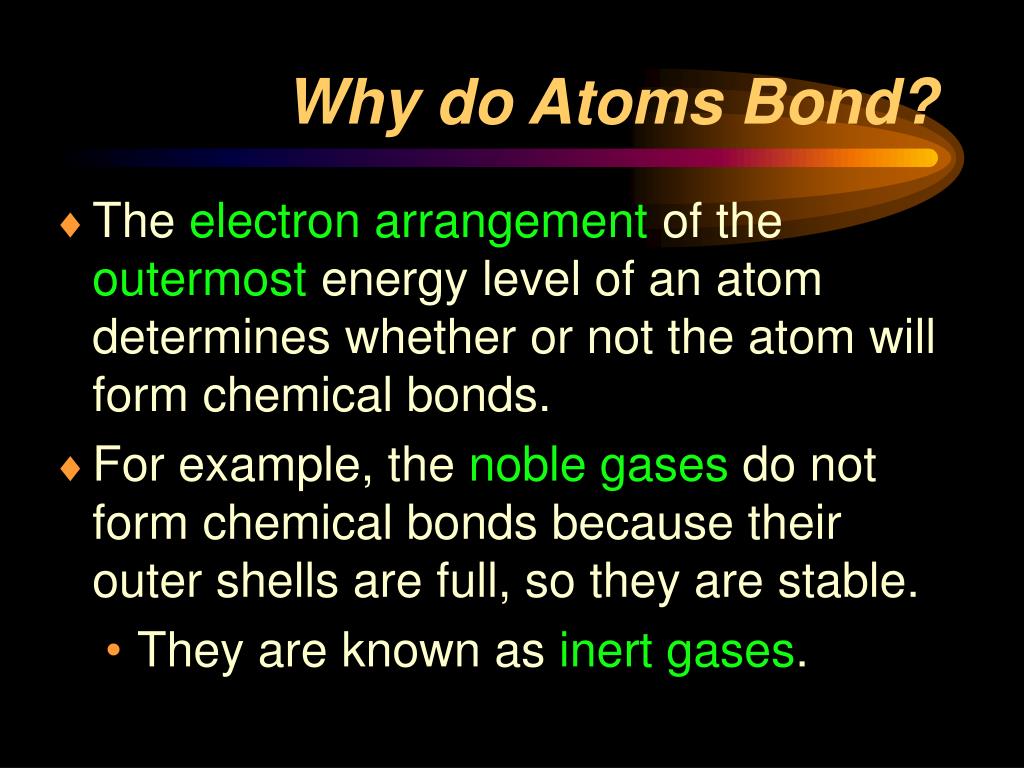
Why Do Atoms Form Chemical Bonds - Web why do atoms bond? Atoms form chemical bonds because they want a lower energy and more stable electron configuration. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of electrons as in covalent bonds. Web bonds form when atoms share or transfer valence electrons. When atoms approach one another, their electrons interact and tend to distribute themselves in space so that the total energy is lower than it would be in any alternative arrangement. The type of chemical bond maximizes the stability of the atoms that form it. Web why do atoms form chemical bonds? Web posted by city chemical on monday, 01 april 2019. Web atoms form chemical bonds to make their outer electron shells more stable. Simply put, atoms form bonds in order to become more stable.
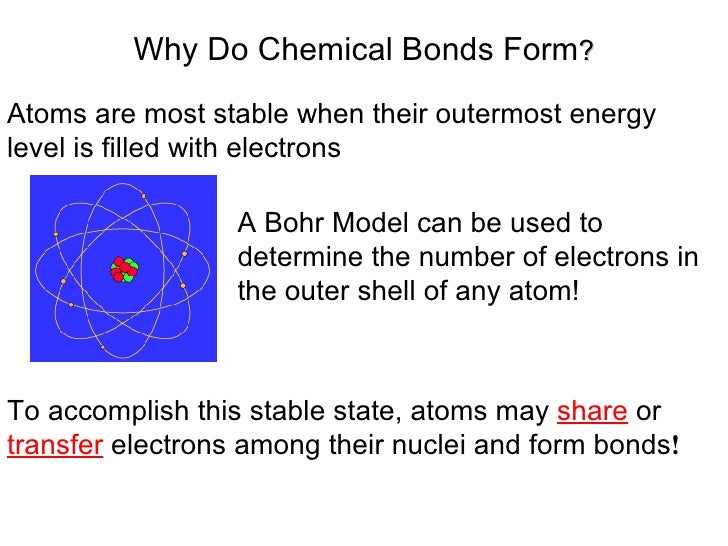
Web bonds form when atoms share or transfer valence electrons. The attraction between different atoms that enables the formation of molecules or compounds. There are three different types of chemical bonds:. Web why form chemical bonds? Web chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other species. Attraction between atoms or ions leads to a chemical bond. Web a chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules, crystals, and other structures. Web atoms form chemical bonds to make their outer electron shells more stable. Atoms form chemical bonds because they want a lower energy and more stable electron configuration. When atoms approach one another, their electrons interact and tend to distribute themselves in space so that the total energy is lower than it would be in any alternative arrangement.
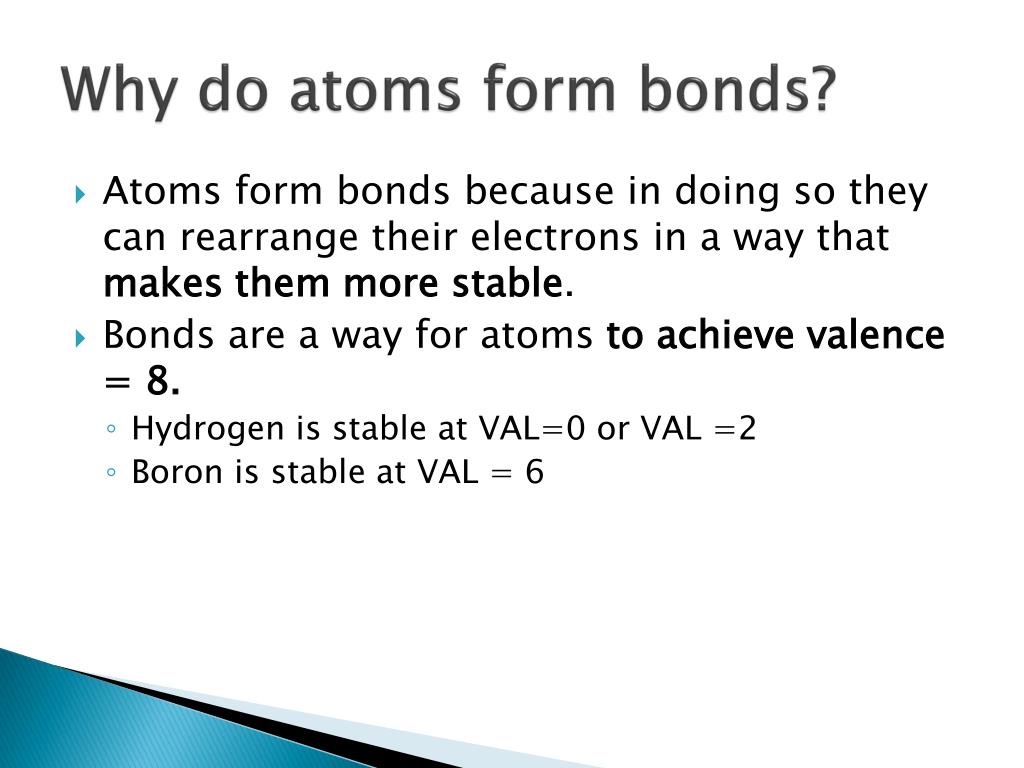
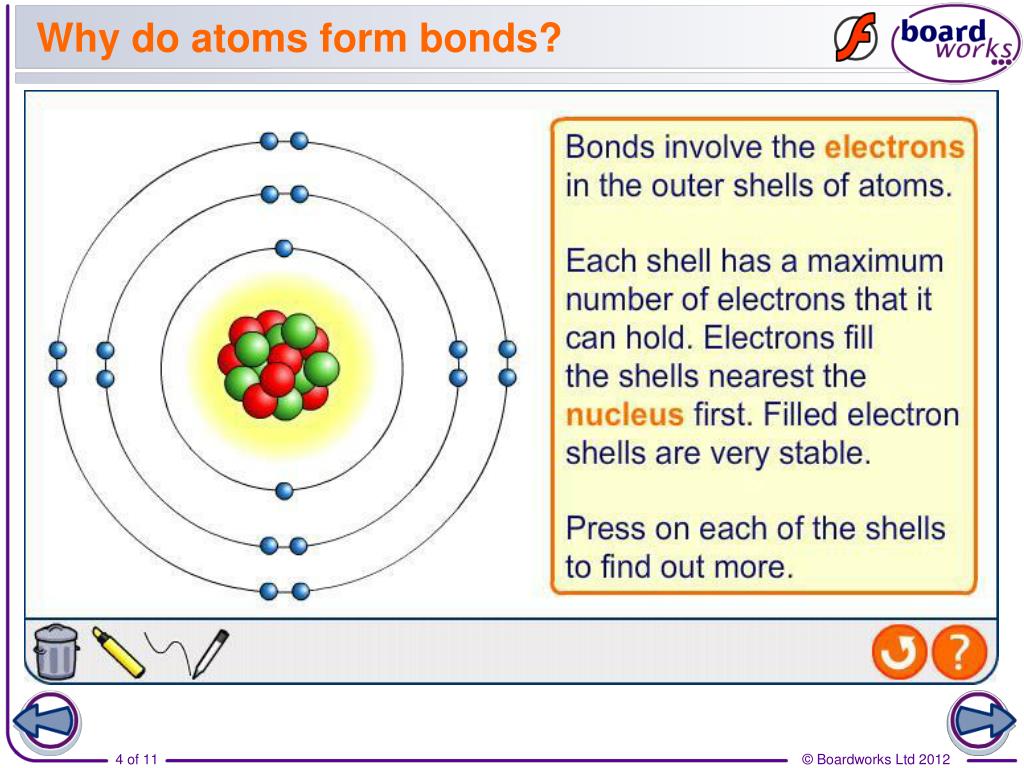
Many atoms become stable when their valence shell is filled with electrons or when they satisfy the. Web why do atoms bond? (often used as a synonym for chemical) a compound. Web a chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules, crystals, and other structures. Attractive forces between atoms that are strong enough to make the linked elements function as a single unit. At the start of this article, we introduced you to a chemical bond: Attraction between atoms or ions leads to a chemical bond. Atoms form chemical bonds because they want a lower energy and more stable electron configuration. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of electrons as in covalent bonds. Simply put, atoms form bonds in order to become more stable.
CHEMISTRY 9TH CH 4, LECTURE 1, Why do Atoms Form Chemical Bonds
Web why do atoms form chemical bonds? Web a chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules, crystals, and other structures. Many atoms become stable when their valence shell is filled with electrons or when they satisfy the. Web why form chemical bonds? Web chemical bonding, any of the interactions that account.
PPT What are bonds? PowerPoint Presentation, free download ID5980343
According to the types of bonds contained in a molecule, the physical properties including melting point, hardness, electrical and thermal conductivity and solubility are determined. Web a chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules, crystals, and other structures. Web why do atoms form chemical bonds? Web bonds form when atoms share.
PPT Chapter 7 Atoms & Bonding PowerPoint Presentation, free download
All bonds appear to link atoms through a sharing of — or an attempt to share — electrons. 149) why does the octet rule not always refer to a stable arrangement of eight valence electrons? (often used as a synonym for chemical) a compound. Web why do atoms form chemical bonds? Web bonds form when atoms share or transfer valence.
Why Do Atoms Form Chemical Bond in Urdu Hindi Lecture /Chemistry for
Atoms form chemical bonds to achieve a full outer energy level, which is the most stable arrangement of electrons. The attraction between different atoms that enables the formation of molecules or compounds. All bonds appear to link atoms through a sharing of — or an attempt to share — electrons. The type of chemical bond maximizes the stability of the.
Characteristics of Chemical Bonds 1. To understand why atoms form bonds
There are three different types of chemical bonds:. According to the types of bonds contained in a molecule, the physical properties including melting point, hardness, electrical and thermal conductivity and solubility are determined. The type of chemical bond maximizes the stability of the atoms that form it. Simply put, atoms form bonds in order to become more stable. Web chemical.
Biochemistry Honors
Simply put, atoms form bonds in order to become more stable. Attraction between atoms or ions leads to a chemical bond. At the start of this article, we introduced you to a chemical bond: Atoms form chemical bonds to achieve a full outer energy level, which is the most stable arrangement of electrons. But why do atoms bond to each.
PPT Chemical Bonding What and Why PowerPoint Presentation, free
Attraction between atoms or ions leads to a chemical bond. Web why do atoms form chemical bonds? Some of the attractive forces are weak, some are very strong. Simply put, atoms form bonds in order to become more stable. Web why do atoms bond?
PPT Ions PowerPoint Presentation, free download ID6738771
Attractive forces between atoms that are strong enough to make the linked elements function as a single unit. Web why form chemical bonds? The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of electrons as in covalent bonds. Attraction between atoms or ions leads to a chemical bond. But.
PPT Atomic structure and chemical bonding PowerPoint Presentation
Web why do atoms form chemical bonds? Web a chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules, crystals, and other structures. Web sep 4, 2021 3.2: According to the types of bonds contained in a molecule, the physical properties including melting point, hardness, electrical and thermal conductivity and solubility are determined. (often.
PPT Why do atoms form bonds? PowerPoint Presentation, free download
Web a chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules, crystals, and other structures. Many atoms become stable when their valence shell is filled with electrons or when they satisfy the. Attractive forces between atoms that are strong enough to make the linked elements function as a single unit. When atoms approach.
But Why Do Atoms Bond To Each Other In This Way?
The attraction between different atoms that enables the formation of molecules or compounds. 149) why does the octet rule not always refer to a stable arrangement of eight valence electrons? Simply put, atoms form bonds in order to become more stable. Atoms form chemical bonds because they want a lower energy and more stable electron configuration.
Web A Chemical Bond Is A Lasting Attraction Between Atoms Or Ions That Enables The Formation Of Molecules, Crystals, And Other Structures.
At the start of this article, we introduced you to a chemical bond: According to the types of bonds contained in a molecule, the physical properties including melting point, hardness, electrical and thermal conductivity and solubility are determined. Many atoms become stable when their valence shell is filled with electrons or when they satisfy the. Web atoms form chemical bonds to make their outer electron shells more stable.
Web Sep 4, 2021 3.2:
Some of the attractive forces are weak, some are very strong. The type of chemical bond maximizes the stability of the atoms that form it. Attraction between atoms or ions leads to a chemical bond. Web chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other species.
Web Why Do Atoms Bond?
(often used as a synonym for chemical) a compound. Web bonds form when atoms share or transfer valence electrons. Atoms form chemical bonds to achieve a full outer energy level, which is the most stable arrangement of electrons. All bonds appear to link atoms through a sharing of — or an attempt to share — electrons.