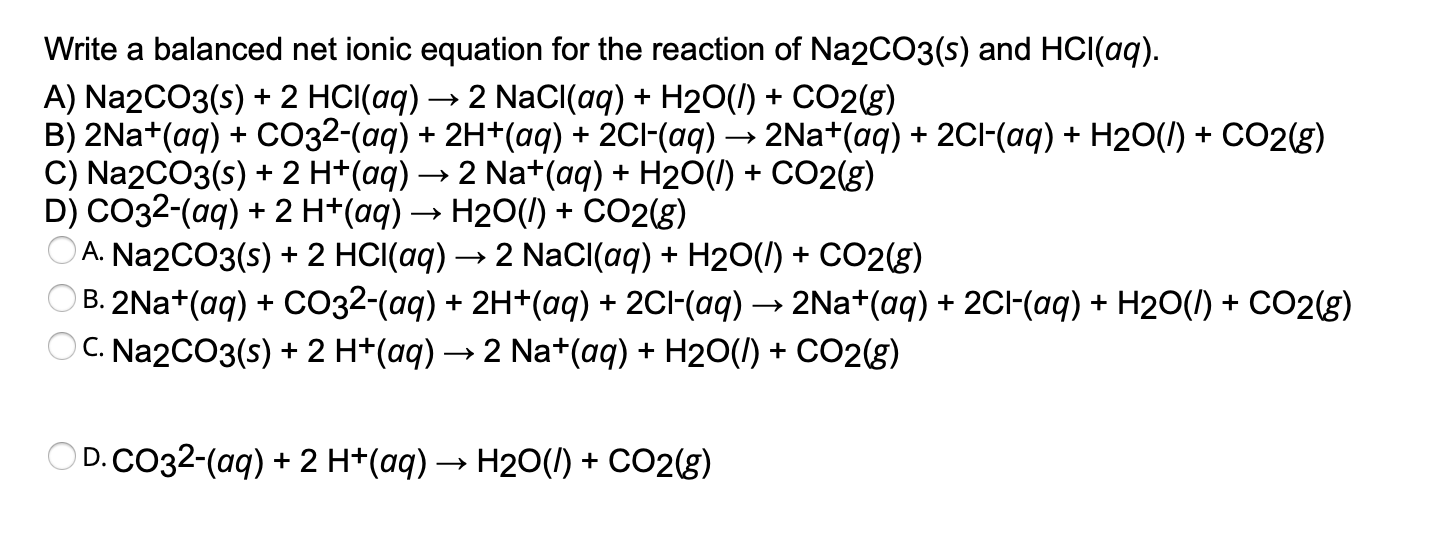Will Hcl And Nacl Form A Buffer
Will Hcl And Nacl Form A Buffer - Web the combination of these two solutes would make a buffer solution. Web naoh + hcl > nacl + h2o b. Hcl + nacl reaction is a. Web why is hcl and nacl not a buffer? Web chemistry chemistry questions and answers which pair of compounds will form a buffer in aqueous solution? Sodium acetate is a salt that dissociates. Hcl dan nacl dicampurkan tidak membentuk larutan penyangga karena berasal dari asam kuat dan garam netral. The weak acid/base therefore shares a common ion with the salt. Even though the second condition for a buffer solution is fulfilled, hcl as a strong. Cuo + h2 > cu + h2o c.
The weak acid/base therefore shares a common ion with the salt. Web they are both salts that will dissociate in water. Web naoh + hcl > nacl + h2o b. Web the hcl + nacl reaction does not form a buffer solution because nacl is salt, and the acidity of hcl is high. Moreover, consider the ionization of water,. Cuo + h2 > cu + h2o c. Na + h2o > naoh + h2 e. Hcl is a strong acid and cannot form buffers. Fe2o3 + co > fe + co2 ahmad77587 may 2021 | 0 replies pada. A) hcl, nacl this contains.
Web why is hcl and nacl not a buffer? Web hcl and nacl are not buffer solutions. Hydrochloric acid (hcl) is a strong acid, not a weak acid, so the combination of these two. Web the combination of these two solutes would make a buffer solution. Hydrochloric acid (hcl) is a strong acid and its conjugate base is the chloride anion provided from sodium. Fe2o3 + co > fe + co2 ahmad77587 may 2021 | 0 replies pada. Web if you dissolve nacl in water you will get some hcl molecules but there's definitely not going to be a significant concentration of hcl formed. Web may 6, 2018. Hcl is a strong acid and cannot form buffers. #1 a buffer is a mixture of a weak acid or weak base and its strong salt.
Tris Buffer 0.1M, pH 7.6 bioWORLD
Hcl is a strong acid and cannot form buffers. Web the overall equation for this reaction is: Web may 6, 2018. Web for example, a buffer can be composed of dissolved acetic acid (hc2h3o2, a weak acid) and sodium acetate ( nac2h3o2). Hcn and nacn hcl and naoh nacn and naoh hcn and hcl hcl.
Mike's Online LabBook The Determination of the Mass of a Product of a
Web naoh + hcl > nacl + h2o b. Mg + hcl > mgcl2 + h2 d. Web hcl and nacl are not buffer solutions. Naoh + hcl → h2o and nacl. Where, ka = [clo−][h +] [h clo] ≈ 3.0 ⋅ 10−8.
Solved Write a balanced net ionic equation for the reaction
Sodium acetate is a salt that dissociates. Web for example, a buffer can be composed of dissolved hc 2 h 3 o 2 (a weak acid) and nac 2 h 3 o 2 (the salt derived from that weak acid). Hydrochloric acid (hcl) is a strong acid, not a weak acid, so the. #1 a buffer is a mixture of.
Relative viscosity of DNA in TrisHCl/NaCl buffer solution (pH 7.2) in
Web the first condition for a buffer solution is not fulfilled. A) hcl, nacl this contains. Hydrochloric acid (hcl) is a strong acid and its conjugate base is the chloride anion provided from sodium. Mg + hcl > mgcl2 + h2 d. Web why is hcl and nacl not a buffer?
NaOH+HCl=NaCl+H2O balance it Brainly.in
Even though the second condition for a buffer solution is fulfilled, hcl as a strong. Web naoh + hcl > nacl + h2o b. Why hcl nacl is not a buffer? Web why is hcl and nacl not a buffer? Sodium acetate is a salt that dissociates.
The catalytic effect of HClNaCl on corn stover. Reaction conditions
Hydrochloric acid (hcl) is a strong acid, not a weak acid, so the. Web may 6, 2018. Cuo + h2 > cu + h2o c. Hcn and nacn hcl and naoh nacn and naoh hcn and hcl hcl. Web we would like to show you a description here but the site won’t allow us.
Best Would Hcl And Nacl Make A Buffer Home & Home
Hcl (g) + nacl (s) → nah (s) + cl 2 (g) a salt (nacl) and water are produced in this reaction when an acid (hcl) reacts with a base (naoh),. A) hcl, nacl this contains. Web for example, a buffer can be composed of dissolved acetic acid (hc2h3o2, a weak acid) and sodium acetate ( nac2h3o2). Web for example,.
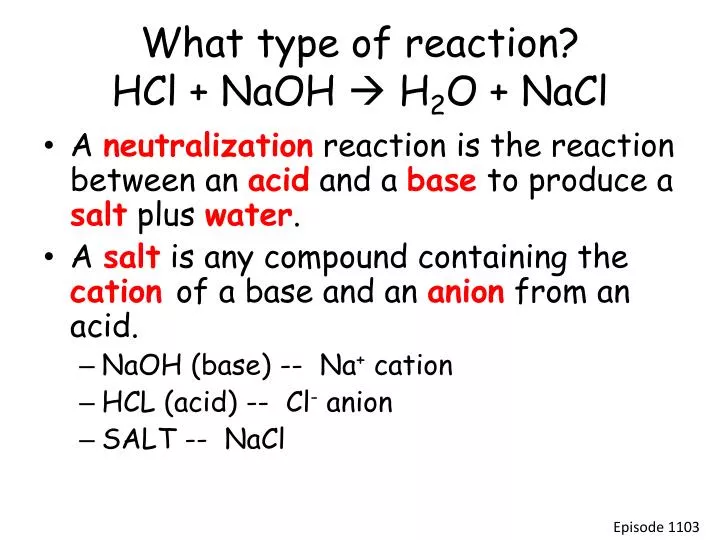
PPT What type of reaction? HCl + NaOH H 2 O + NaCl PowerPoint
Web the hcl + nacl reaction does not form a buffer solution because nacl is salt, and the acidity of hcl is high. Naoh + hcl → h2o and nacl. Cuo + h2 > cu + h2o c. The weak acid/base therefore shares a common ion with the salt. Hcl is a strong acid and cannot form buffers.
Discrepant Event
Hcl (g) + nacl (s) → nah (s) + cl 2 (g) a salt (nacl) and water are produced in this reaction when an acid (hcl) reacts with a base (naoh),. Web we would like to show you a description here but the site won’t allow us. Web why is hcl and nacl not a buffer? The weak acid/base therefore.
How to Balance Na2CO3 + HCl = NaCl + H2O + CO2 (Sodium carbonate
Web may 6, 2018. Hcl + nacl reaction is a. Hcl dan nacl dicampurkan tidak membentuk larutan penyangga karena berasal dari asam kuat dan garam netral. The weak acid/base therefore shares a common ion with the salt. Web chemistry chemistry questions and answers which pair of compounds will form a buffer in aqueous solution?
The Weak Acid/Base Therefore Shares A Common Ion With The Salt.
Consider the buffer system's equilibrium, h clo ⇌ clo− + h +. Hcl is a strong acid and cannot form buffers. Hydrochloric acid (hcl) is a strong acid, not a weak acid, so the. Hcl + nacl reaction is a.
Web Why Is Hcl And Nacl Not A Buffer?
Hydrochloric acid (hcl) is a strong acid and its conjugate base is the chloride anion provided from sodium. Cuo + h2 > cu + h2o c. Web the combination of these two solutes would make a buffer solution. Web we would like to show you a description here but the site won’t allow us.
Web For Example, A Buffer Can Be Composed Of Dissolved Acetic Acid (Hc2H3O2, A Weak Acid) And Sodium Acetate ( Nac2H3O2).
Web they are both salts that will dissociate in water. Even though the second condition for a buffer solution is fulfilled, hcl as a strong. Why hcl nacl is not a buffer? Mg + hcl > mgcl2 + h2 d.
Web For Example, A Buffer Can Be Composed Of Dissolved Hc 2 H 3 O 2 (A Weak Acid) And Nac 2 H 3 O 2 (The Salt Derived From That Weak Acid).
Web given in the question we have a combination of strong acid $hcl$ and a salt of strong acid and strong base $nacl(hcl + naoh)$ thus this combination cannot act as. Web if you dissolve nacl in water you will get some hcl molecules but there's definitely not going to be a significant concentration of hcl formed. Hcl dan nacl dicampurkan tidak membentuk larutan penyangga karena berasal dari asam kuat dan garam netral. Web hcl and nacl are not buffer solutions.