Can Nitrogen Form 4 Bonds
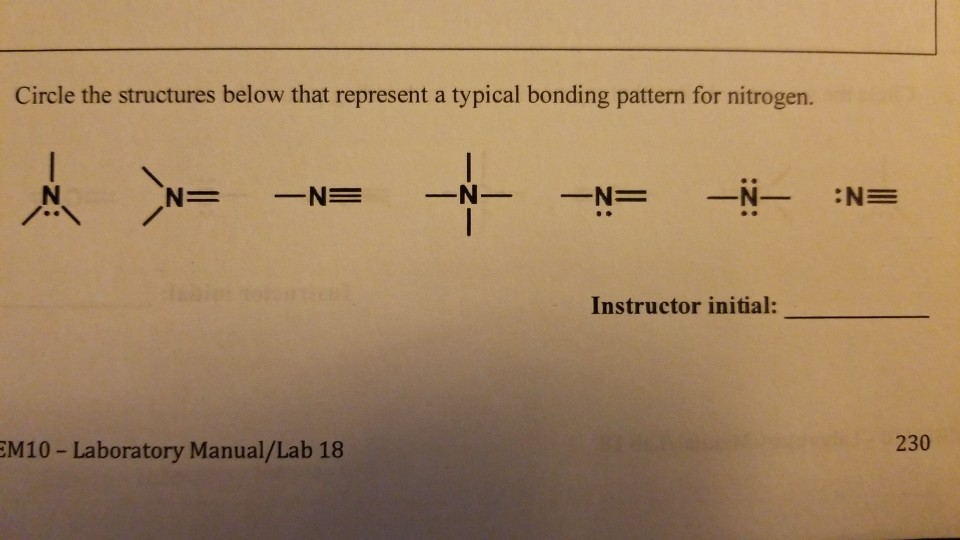
Can Nitrogen Form 4 Bonds - Web carbon has six electrons (two core and four valence) and can form four bonds with neighboring atoms. Web nitrogen will usually have 3 bonds, occasionally 4; Thus, there is a lot of energy in the compounds of. If you look at the above image you can see that when nitrogen has a positive charge (one less electron), it can form four. It can donate this electron pair to form a coordinate bond. Methane, ( ch 4, is a single carbon atom covalently bonded to four hydrogen. Web ammonia, ( nh 3, is a central nitrogen atom bonded to three hydrogen atoms. Andreas grohmann, juergen riede and hubert schmidbauer at the technical. Web nitrogen can have 4 bonds, but it prefers to have 3. However, in the case of n20, n forms a triple bond with the second n (the central atom) and that second.
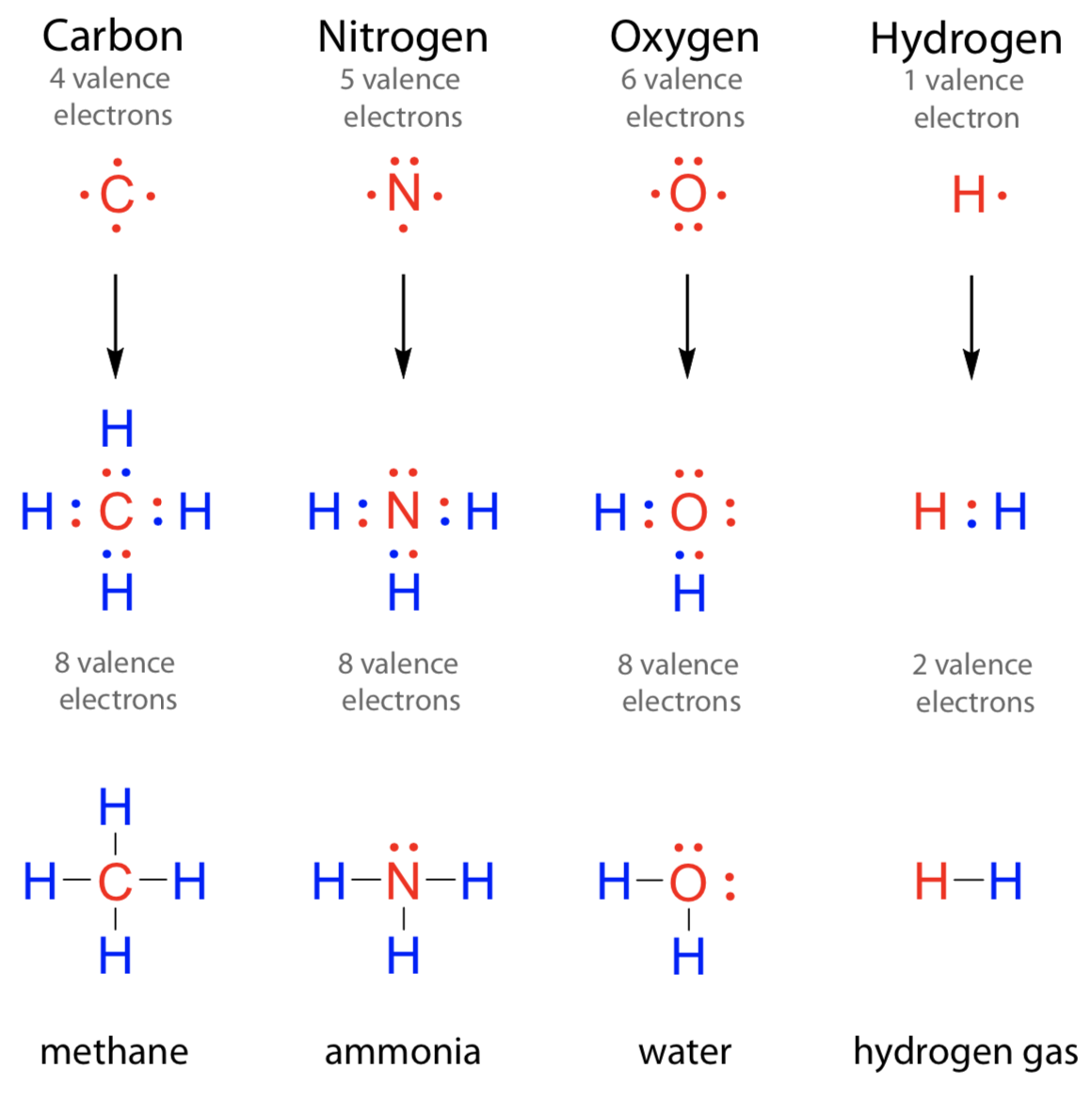
Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ch 4 (methane). Web nitrogen will usually have 3 bonds, occasionally 4; Web can nitrogen form 4 bonds. In diazonium ion n has 4 bonds, then one of those 4. Nitrogen can also have 2 bonds if the nitrogen atom is negatively. This coordinate bond that nitrogen forms by. Group 5a (15) elements such as nitrogen. Solution suggest corrections 1 similar questions q. Web nitrogen atoms will form three covalent bonds (also called triple covalent) between two atoms of nitrogen because each nitrogen atom needs three electrons to fill its. Web why nitrogen can form 4 bonds?
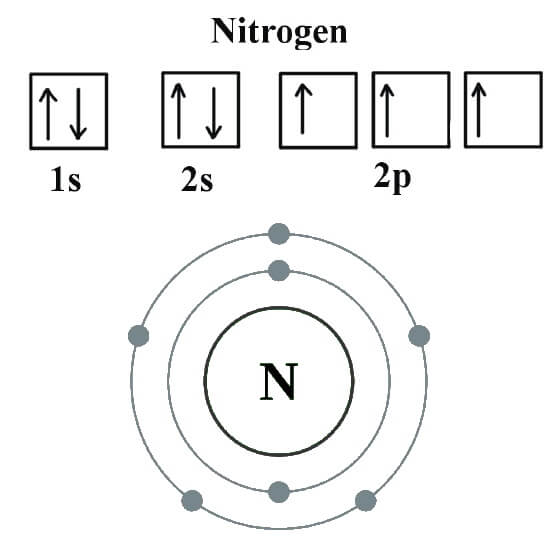
Andreas grohmann, juergen riede and hubert schmidbauer at the technical. Web ammonia, ( nh 3, is a central nitrogen atom bonded to three hydrogen atoms. Group 5a (15) elements such as nitrogen. Thus, there is a lot of energy in the compounds of. In diazonium ion n has 4 bonds, then one of those 4. Web if you look at the above image you can see that when nitrogen has a positive charge (one less electron), it can form four covalent bonds. It can donate this electron pair to form a coordinate bond. Web nitrogen has seven electrons (2 core and 5 valence) (1s2, 2s2, 2px1, 2py1, 2pz1). But it can't have 4. Web carbon has six electrons (two core and four valence) and can form four bonds with neighboring atoms.
How Many Bonds Can Nitrogen Form Jacks Of Science
Web nitrogen's valenc number is 3. Nitrogen ( n 2) reacts with oxygen ( o2 ), the compound n o2 can be formed as a. Solution suggest corrections 1 similar questions q. Web if you look at the above image you can see that when nitrogen has a positive charge (one less electron), it can form four covalent bonds. Web.
Why does nitrogen form 4 bonds and oxgen 3 bonds. Can someone explain
Nitrogen ( n 2) reacts with oxygen ( o2 ), the compound n o2 can be formed as a. In diazonium ion n has 4 bonds, then one of those 4. Carbon can form four covalent bonds to create an organic molecule. Web i was told that nitrogen can form only up to three bonds. If you look at the.
Solved How many bonds does nitrogen typically form? Does
Group 5a (15) elements such as nitrogen. But it can't have 4. Web nitrogen has seven electrons (2 core and 5 valence) (1s2, 2s2, 2px1, 2py1, 2pz1). Andreas grohmann, juergen riede and hubert schmidbauer at the technical. Nitrogen ( n 2) reacts with oxygen ( o2 ), the compound n o2 can be formed as a.
Can nitrogen form 4 bonds.
Web i was told that nitrogen can form only up to three bonds. Andreas grohmann, juergen riede and hubert schmidbauer at the technical. Group 5a (15) elements such as nitrogen. However, if the n has 4 bonds it will be positively charged. Web nitrogen's valenc number is 3.
SOLID NITROGEN YouTube
Group 5a (15) elements such as nitrogen. Nitrogen can also have 2 bonds if the nitrogen atom is negatively. Group 5a (15) elements such as nitrogen. Two core and five valence: However, if the n has 4 bonds it will be positively charged.
Nitrogen from rock could fuel more plant growth around the world but
Web carbon has six electrons (two core and four valence) and can form four bonds with neighboring atoms. Solution suggest corrections 1 similar questions q. Thus, there is a lot of energy in the compounds of. Andreas grohmann, juergen riede and hubert schmidbauer at the technical. However, in the case of n20, n forms a triple bond with the second.
10 Interesting Nitrogen Facts My Interesting Facts
Web nitrogen atoms will form three covalent bonds (also called triple covalent) between two atoms of nitrogen because each nitrogen atom needs three electrons to fill its. Web i was told that nitrogen can form only up to three bonds. Web nitrogen will usually have 3 bonds, occasionally 4; Web why nitrogen can form 4 bonds? So if you are.
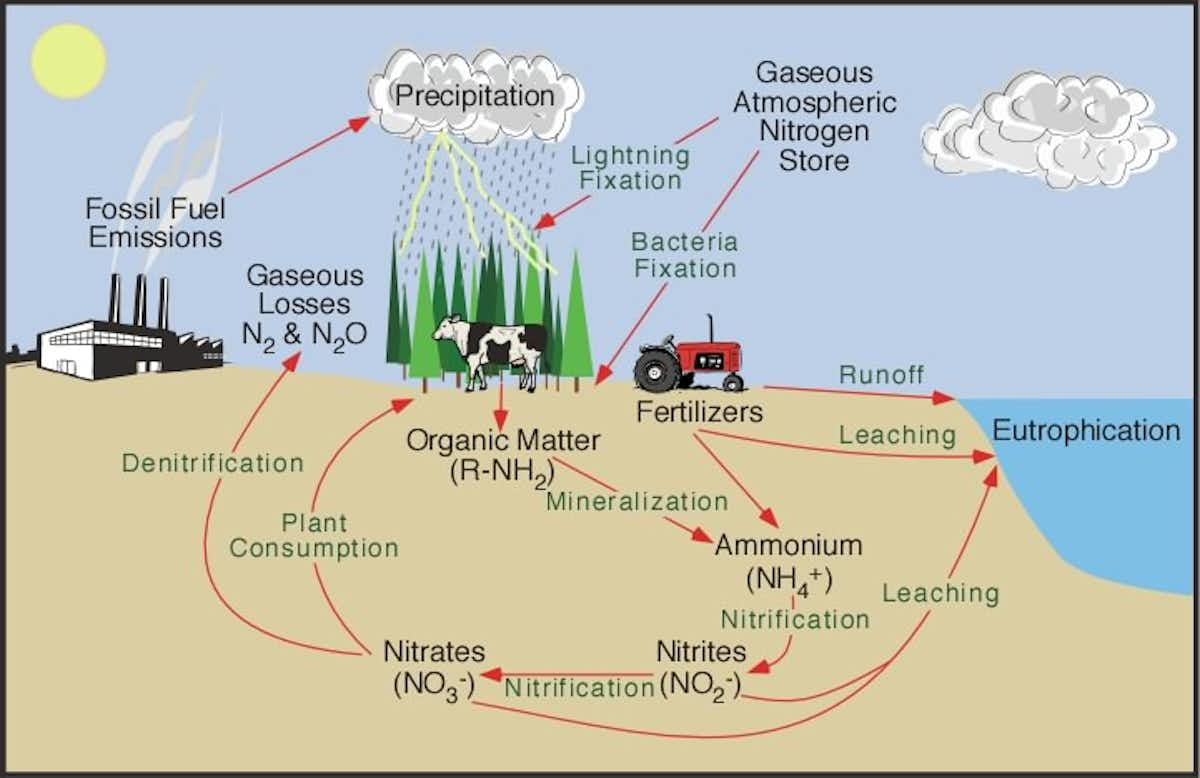
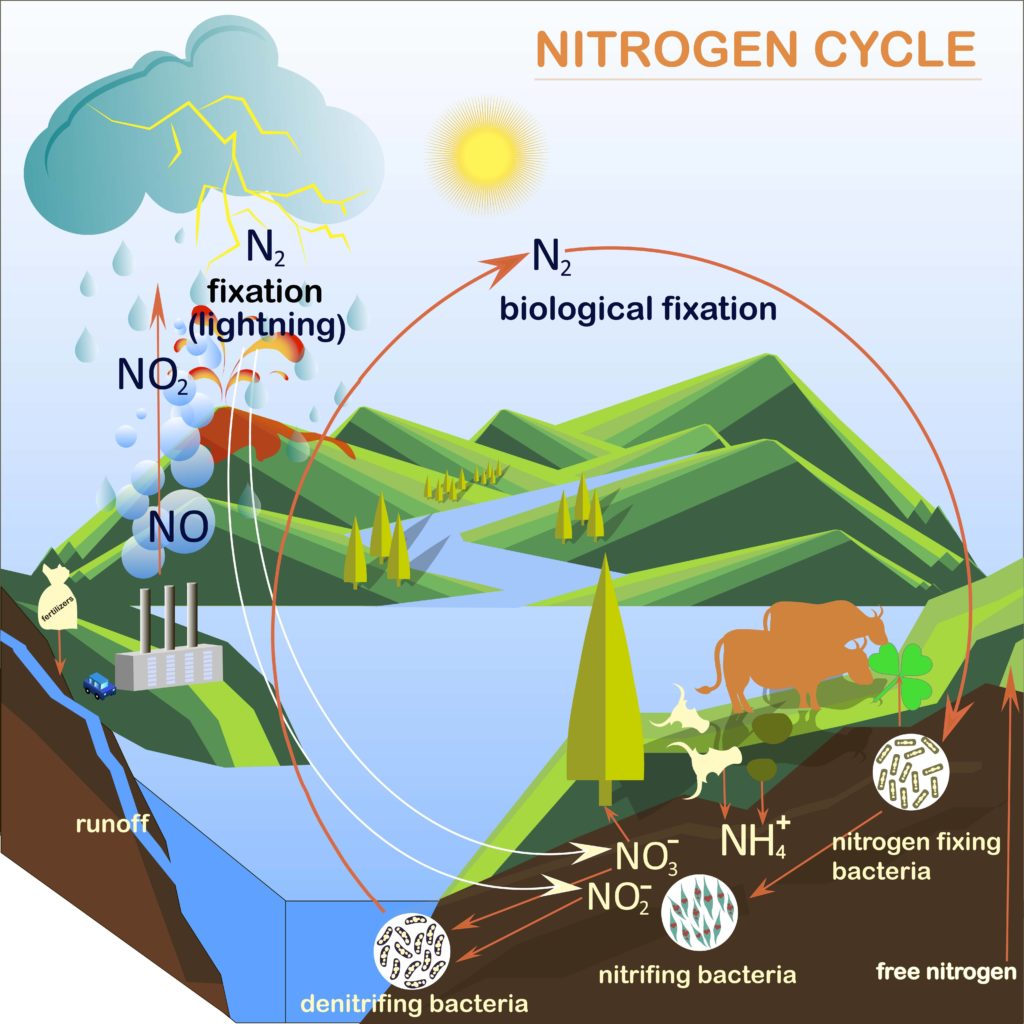
What is the Nitrogen Cycle? Science for Kids
Web nitrogen will usually have 3 bonds, occasionally 4; Two core and five valence: Web nitrogen has one lone pair of electrons in its 2s orbital. In diazonium ion n has 4 bonds, then one of those 4. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ch 4 (methane).
LabXchange
So if you are following the rules, you might well assume that nitrogen would be able to form five. Two core and five valence: Nitrogen can also have 2 bonds if the nitrogen atom is negatively. If you look at the above image you can see that when nitrogen has a positive charge (one less electron), it can form four..
How many valence electrons does nitrogen have? Ask4Essay
Web nitrogen has seven electrons (2 core and 5 valence) (1s2, 2s2, 2px1, 2py1, 2pz1). Group 5a (15) elements such as nitrogen. Web nitrogen will usually have 3 bonds, occasionally 4; Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ch 4 (methane). Nitrogen ( n 2) reacts with oxygen (.
Web Nitrogen Has One Lone Pair Of Electrons In Its 2S Orbital.
Web nitrogen atom can attain an octet configuration by sharing three electrons with another nitrogen atom, forming a triple bond (three pairs of electrons shared) diagram of. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ch 4 (methane). So if it has 4 bonds it'll have a charge of +1 (b/c it has 1 more bond than its preferred #). Web why nitrogen can form 4 bonds?
Web Can Nitrogen Form 4 Bonds.
In diazonium ion n has 4 bonds, then one of those 4. Group 5a (15) elements such as nitrogen. However, if the n has 4 bonds it will be positively charged. If you look at the above image you can see that when nitrogen has a positive charge (one less electron), it can form four.
Web According To The Textbooks, A Nitrogen Atom Cannot Form More Than Four Bonds.
Web ammonia, ( nh 3, is a central nitrogen atom bonded to three hydrogen atoms. This coordinate bond that nitrogen forms by. Web i was told that nitrogen can form only up to three bonds. Web nitrogen atoms will form three covalent bonds (also called triple covalent) between two atoms of nitrogen because each nitrogen atom needs three electrons to fill its.
Methane, ( Ch 4, Is A Single Carbon Atom Covalently Bonded To Four Hydrogen.
Carbon can form four covalent bonds to create an organic molecule. Group 5a (15) elements such as nitrogen. Two core and five valence: Nitrogen ( n 2) reacts with oxygen ( o2 ), the compound n o2 can be formed as a.