Iron Reacts With Oxygen To Form Iron Iii Oxide
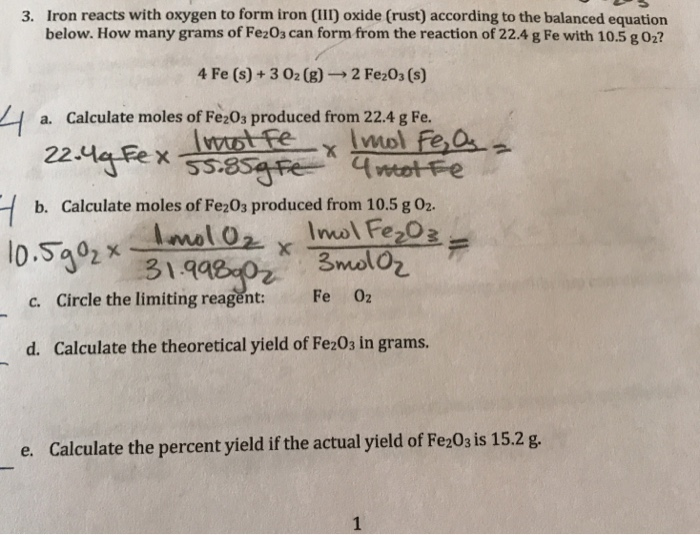
Iron Reacts With Oxygen To Form Iron Iii Oxide - 4fe (s)+3o2 (g) 2fe2o3 (s)4fe (s)+3o2 (g) 2fe2o3 (s) suppose 21.2 g21.2 g of. When iron rusts, solid iron reacts with gaseous oxygen to form solid iron (iii) oxide. Web ron reacts with oxygen at high temperatures to form iron (iii) oxide. Here is the word equation for the reaction: Web the iron reacts with water and oxygen to form hydrated iron (iii) oxide, which we see as rust. Web chemistry chemistry questions and answers write balanced equations for the chemical reactions described. Three different levels of interpretation in science sorting and classifying, interpreting, identifying optional (revision) activity:. 4fe (s)+3o2 (g) 2fe2o3 (s) suppose 13.0 g of iron (fe) is reacted with 19.7 g of oxygen. Web this reaction can be written in words as iron iii combining with oxygen gas to form iron iii oxide. Afe +302256 b) if 4.0g iron completely reacted, how many.
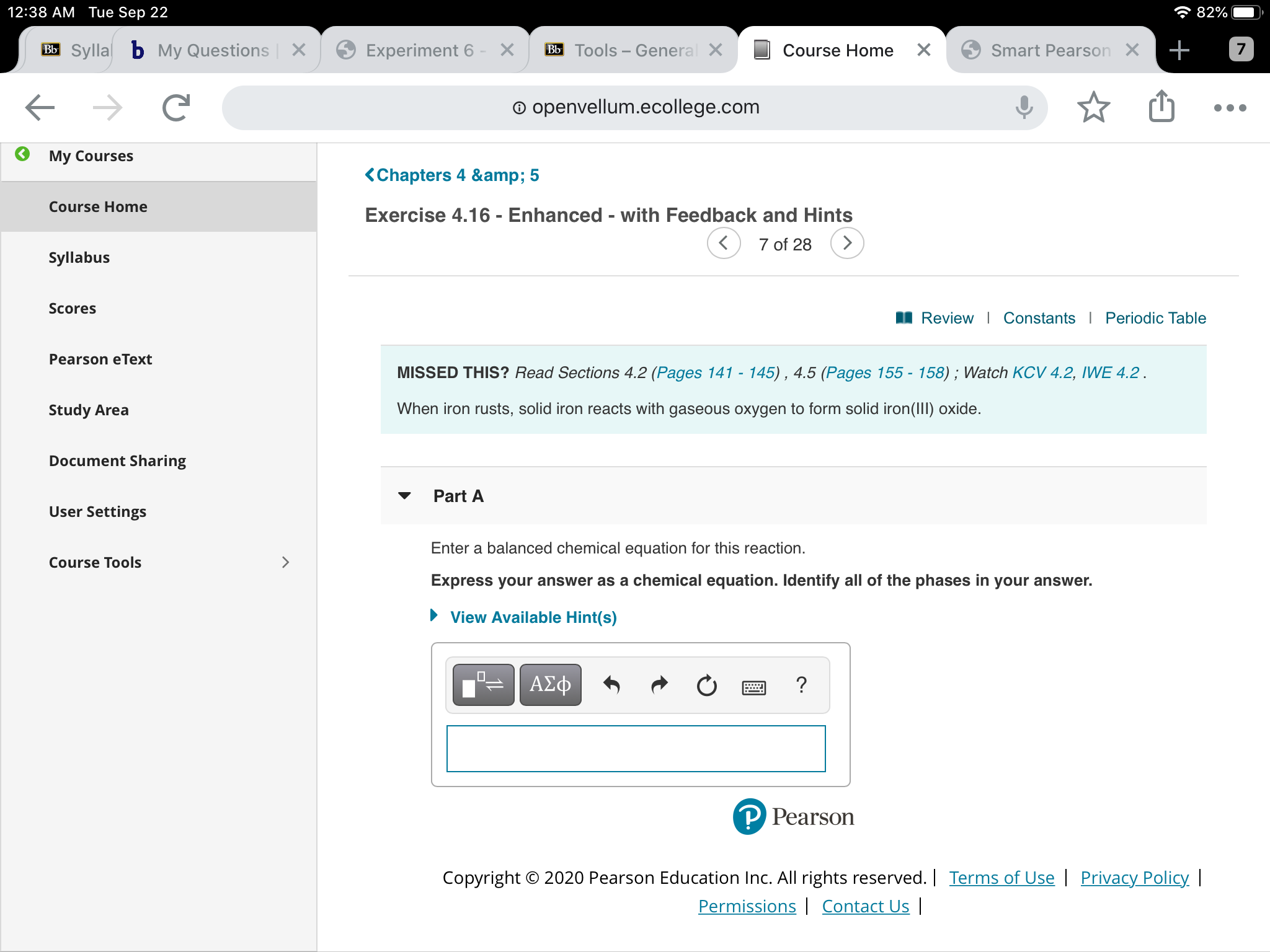
Here is the word equation for the reaction: Enter a balanced chemical equation for this reaction. Web 3.1 the reaction of iron with oxygen (1 hour) activity: Iron reacts with oxygen to form iron (iii) oxide. Web ron reacts with oxygen at high temperatures to form iron (iii) oxide. Afe +302256 b) if 4.0g iron completely reacted, how many. Web iron reacts with oxygen to form iron (iii) oxide: Study guides chemistry 19 cards to name a. Web iron can react with oxygen to form two of its oxides, iron (ii, iii) oxide and iron (iii) oxide. A) write a balanced chemical equation for the reaction.
Iron reacts with oxygen to form iron (iii) oxide. Web this reaction can be written in words as iron iii combining with oxygen gas to form iron iii oxide. A) write a balanced chemical equation for the reaction. It can be prepared in the laboratory by electrolyzing a solution of sodium bicarbonate, an inert electrolyte, with an iron anode: Iron + water + oxygen → hydrated iron. Web iron can react with oxygen to form two of its oxides, iron (ii, iii) oxide and iron (iii) oxide. Enter a balanced chemical equation for this reaction. Web 3.1 the reaction of iron with oxygen (1 hour) activity: Afe +302256 b) if 4.0g iron completely reacted, how many. When iron rusts, solid iron reacts with gaseous oxygen to form solid iron (iii) oxide.
Answered When iron rusts, solid iron reacts with… bartleby
Iron reacts with oxygen to form solid iron (iii) oxide. Iron reacts with oxygen to form iron (iii) oxide. Enter a balanced chemical equation for this reaction. Web the iron reacts with water and oxygen to form hydrated iron (iii) oxide, which we see as rust. When iron rusts, solid iron reacts with gaseous oxygen to form solid iron (iii).
Spice of Lyfe Chemical Equation For Rusting Of Iron
4fe (s)+3o2 (g) 2fe2o3 (s) suppose 13.0 g of iron (fe) is reacted with 19.7 g of oxygen. Assuming there is an excess of iron, how many moles of oxygen. Afe +302256 b) if 4.0g iron completely reacted, how many. Iron reacts with oxygen at high temperatures to form iron (iii) oxide. Web the iron reacts with water and oxygen.
SOLVEDIron metal reacts with oxygen to give iron…
You know it is iron iii, not iron ii, because oxygen is always. Study guides chemistry 19 cards to name a. Iron + water + oxygen → hydrated iron. 4fe (s)+3o2 (g) 2fe2o3 (s)4fe (s)+3o2 (g) 2fe2o3 (s) suppose 21.2 g21.2 g of. Web chemistry chemistry questions and answers write balanced equations for the chemical reactions described.
when iron rusts, solid iron reacts with gaseous oxygen to form solid
A) write a balanced chemical equation for the reaction. Web 3.1 the reaction of iron with oxygen (1 hour) activity: 4fe (s)+3o2 (g) 2fe2o3 (s) suppose 13.0 g of iron (fe) is reacted with 19.7 g of oxygen. Iron reacts with oxygen to form iron (iii) oxide. Web when two smaller substances combine to form only one product it is.
[Solved] Solid Iron (III) reacts with oxygen gas to form iron (III
Enter a balanced chemical equation for this reaction. Web 3.1 the reaction of iron with oxygen (1 hour) activity: Web ron reacts with oxygen at high temperatures to form iron (iii) oxide. Web iron reacts with oxygen to form iron (iii) oxide: Iron reacts with oxygen to form solid iron (iii) oxide.
PPT The Law of Conservation of Matter PowerPoint Presentation ID
Web ron reacts with oxygen at high temperatures to form iron (iii) oxide. Iron reacts with oxygen to form iron (iii) oxide. Web 3.1 the reaction of iron with oxygen (1 hour) activity: A) write a balanced chemical equation for the reaction. Web chemistry chemistry questions and answers write balanced equations for the chemical reactions described.
Solved Iron reacts with oxygen to produce iron (III)
Upon reacting with oxygen, iron will be oxidized to either the +3. Web 3.1 the reaction of iron with oxygen (1 hour) activity: 4fe (s)+3o2 (g) 2fe2o3 (s) suppose 13.0 g of iron (fe) is reacted with 19.7 g of oxygen. Enter a balanced chemical equation for this reaction. Web iron reacts with oxygen to form iron (iii) oxide:
Solved 3. Iron reacts with oxygen to form iron (III) oxide
Web the iron reacts with water and oxygen to form hydrated iron (iii) oxide, which we see as rust. Web iron can react with oxygen to form two of its oxides, iron (ii, iii) oxide and iron (iii) oxide. When iron rusts, solid iron reacts with gaseous oxygen to form solid iron (iii) oxide. Iron reacts with oxygen to form.
Rusting Equation FOTO IMAGES
Iron reacts with oxygen at high temperatures to form iron (iii) oxide. Web ron reacts with oxygen at high temperatures to form iron (iii) oxide. You know it is iron iii, not iron ii, because oxygen is always. Web the iron reacts with water and oxygen to form hydrated iron (iii) oxide, which we see as rust. Three different levels.
Characteristics of iron and its reaction with oxygen MEL Chemistry
It can be prepared in the laboratory by electrolyzing a solution of sodium bicarbonate, an inert electrolyte, with an iron anode: Web when two smaller substances combine to form only one product it is usually a synthesis, or combination reaction. Upon reacting with oxygen, iron will be oxidized to either the +3. Enter a balanced chemical equation for this reaction..
Web Ron Reacts With Oxygen At High Temperatures To Form Iron (Iii) Oxide.
Three different levels of interpretation in science sorting and classifying, interpreting, identifying optional (revision) activity:. Study guides chemistry 19 cards to name a. Web the iron reacts with water and oxygen to form hydrated iron (iii) oxide, which we see as rust. Afe +302256 b) if 4.0g iron completely reacted, how many.
Web Iron Can React With Oxygen To Form Two Of Its Oxides, Iron (Ii, Iii) Oxide And Iron (Iii) Oxide.
Assuming there is an excess of iron, how many moles of oxygen. Web iron reacts with oxygen to form iron (iii) oxide: Iron + oxygen + water → hydrated iron(iii) oxide Web 3.1 the reaction of iron with oxygen (1 hour) activity:
A) Write A Balanced Chemical Equation For The Reaction.
Iron + water + oxygen → hydrated iron. Web this reaction can be written in words as iron iii combining with oxygen gas to form iron iii oxide. Web when two smaller substances combine to form only one product it is usually a synthesis, or combination reaction. Enter a balanced chemical equation for this reaction.
4Fe (S)+3O2 (G) 2Fe2O3 (S) Suppose 13.0 G Of Iron (Fe) Is Reacted With 19.7 G Of Oxygen.
Iron reacts with oxygen to form solid iron (iii) oxide. Iron reacts with oxygen to form iron (iii) oxide. 4fe (s)+3o2 (g) 2fe2o3 (s)4fe (s)+3o2 (g) 2fe2o3 (s) suppose 21.2 g21.2 g of. Iron reacts with oxygen at high temperatures to form iron (iii) oxide.

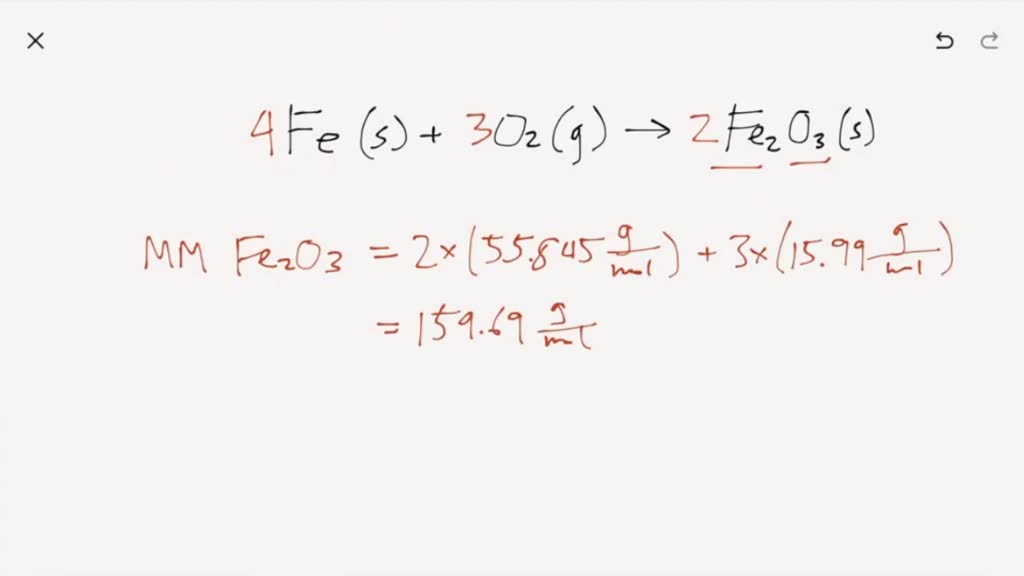



:max_bytes(150000):strip_icc()/BalanceEquations1-56a132765f9b58b7d0bcf535.png)
